Question
Decane has a freezing point of –30 °C and a boiling point of 174 °C.
A small sample of decane is placed in an open beaker in an oven at a temperature of 120 °C and
at atmospheric pressure for 24 hours.
What happens to the sample of decane?
A It boils.
B It evaporates.
C It melts.
D It sublimes.
Answer/Explanation
Ans: B
Question
A student put exactly 25.00 \(cm^3\) of dilute hydrochloric acid into a conical flask.
The student added 2.5 g of solid sodium carbonate and measured the change in temperature of
the mixture.
Which apparatus does the student need to use?
A balance, measuring cylinder, thermometer
B balance, pipette, stopwatch
C balance, pipette, thermometer
D burette, pipette, thermometer
Answer/Explanation
Ans: C
Question
A student separates sugar from pieces of broken glass by dissolving the sugar in water and
filtering off the broken glass.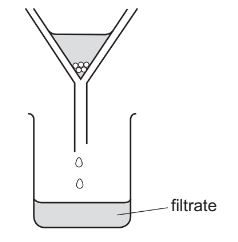
What is the filtrate?
A broken glass only
B broken glass and sugar solution
C pure water
D sugar solution
Answer/Explanation
Ans: D
Question
Which statement explains why metals conduct electricity when solid?
A They have atoms which are free to move.
B They have electrons which are free to move.
C They have molecules which are free to move.
D They have positive ions which are free to move.
Answer/Explanation
Ans: B
Question
Which description of brass is correct?
A alloy
B compound
C element
D non-metal
Answer/Explanation
Ans: A
Question
The equation for the reaction of iron(III) oxide with carbon monoxide is shown.
\(Fe_2O_3(s) + 3CO(g) → 2Fe(s) + 3CO_2(g)\)
What is the maximum mass of iron that can be made from 480 g of iron(III) oxide?
A 56 g B 112 g C 168 g D 336 g
Answer/Explanation
Ans: D
Question
Which statement describes the attractive forces between molecules?
A They are strong covalent bonds which hold molecules together.
B They are strong ionic bonds which hold molecules together.
C They are weak forces formed between covalently-bonded molecules.
D They are weak forces which hold ions together in a lattice.
Answer/Explanation
Ans: C
Question
Which statement about carbon is correct?
A Diamond and graphite both have simple molecular structures.
B Diamond and graphite are both used to make cutting tools.
C Each carbon atom in diamond is bonded to three other carbon atoms.
D Graphite conducts electricity and has a giant covalent structure.
Answer/Explanation
Ans: D
Question
The formula of an aluminium ion is \(Al^{3+}\).
What is the formula of aluminium sulfate?
A \(Al_2SO_4\) B \(Al(SO_4)_2\) C \(Al_2(SO_4)_3\) D \(Al_3(SO_4)_2\)
Answer/Explanation
Ans: C
Question
Which statements about the products of electrolysis, using inert electrodes, are correct?
1 When molten lead(II) bromide is electrolysed, bromine is formed at the cathode.
2 When dilute sulfuric acid is electrolysed, oxygen is formed at the anode.
3 When concentrated aqueous sodium chloride is electrolysed, sodium is formed at
the cathode.
4 When concentrated hydrochloric acid is electrolysed, chlorine is formed at the
anode.
A 1 and 2 B 1 and 3 C 2 and 4 D 3 and 4
Answer/Explanation
Ans: C
Question
Chlorine reacts with ethane to produce chloroethane and hydrogen chloride.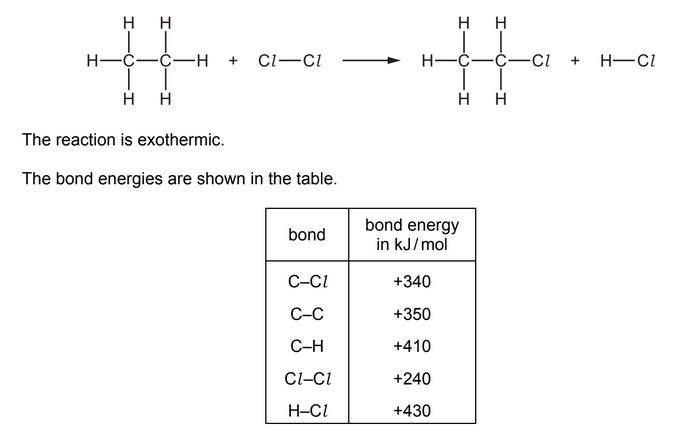
What is the energy change for the reaction?
A –1420 kJ /mol
B –120 kJ /mol
C +120 kJ / mol
D +1420 kJ / mol
Answer/Explanation
Ans: B
Question
Hydrogen is used as a fuel in rockets and is also used in hydrogen fuel cells.
Which statements are correct?
1 Both uses produce water vapour.
2 Burning hydrogen produces polluting gases.
3 A fuel cell produces electricity.
A 1, 2 and 3 B 1 and 3 only C 1 only D 2 and 3 only
Answer/Explanation
Ans: B
Question
Which statements about the effect of increasing the temperature on the rate of a reaction are correct?
1 It increases the rate of a reaction.
2 It increases the activation energy.
3 It increases the frequency of collisions.
A 1, 2 and 3 B 1 and 2 only C 1 and 3 only D 2 and 3 only
Answer/Explanation
Ans: C
Question
Ammonia is made by reacting nitrogen with hydrogen.
The equation for the reaction is shown.
\(N_2(g) + 3H_2(g) 2NH_3(g)\)
The forward reaction is exothermic.
Which changes in temperature and pressure decrease the yield of ammonia?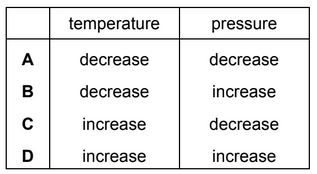
Answer/Explanation
Ans: C
Question
X is a pink solid.
Y is a blue solid.
When X is heated, water is produced and the solid turns blue.
When water is added to Y, the solid turns pink.
What are X and Y?
Answer/Explanation
Ans: B
Question
Iron(II) chloride solution reacts with chlorine gas.
The equation is shown.
\(2FeCl_2(aq) + Cl_2(g) → 2FeCl_3(aq)\)
Which statements about this reaction are correct?
1 \(Fe^{2+}\) ions are reduced to \(Fe^{3+}\) ions.
2 Chlorine acts as a reducing agent.
3 \(Fe^{2+}\) ions each lose an electron.
4 \(Cl_2\) molecules are reduced to \(Cl^–\) ions.
A 1 and 2 B 2 and 3 C 2 and 4 D 3 and 4
Answer/Explanation
Ans: D
Question
Which row describes the properties of an acid?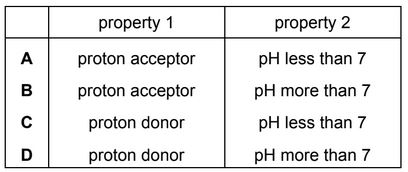
Answer/Explanation
Ans: C
Question
Which element forms an amphoteric oxide?
A aluminium
B carbon
C magnesium
D silicon
Answer/Explanation
Ans: A
Question
Copper(II) chloride crystals are made by adding solid copper(II) carbonate to dilute
hydrochloric acid until no more dissolves.
Which process is used to obtain pure copper(II) chloride crystals from the mixture?
A distillation of the mixture
B evaporation of the mixture
C filtration followed by drying of the residue
D filtration followed by evaporation of the filtrate
Answer/Explanation
Ans: D
Question
Moving from right to left across the Periodic Table the elements show increasing metallic character.
Why does metallic character increase from right to left across a period?
A The atoms have more electrons in their outer shells.
B The atoms more readily gain electrons to form negative ions.
C The atoms more readily lose electrons to form positive ions.
D The charge on the nucleus of each atom gets larger.
Answer/Explanation
Ans: C
Question
A period of the Periodic Table is shown.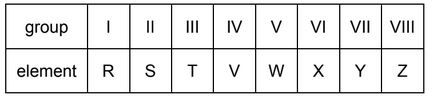
The letters are not their chemical symbols.
Which statement is correct?
A Element R does not conduct electricity.
B Elements R and Y react together to form an ionic compound.
C Element Z exists as a diatomic molecule.
D Element Z reacts with element T.
Answer/Explanation
Ans: B
Question
Group VII elements show trends in their physical properties going down the group.
Answer/Explanation
Ans: B
Question
Some properties of two metals, G and H, are shown.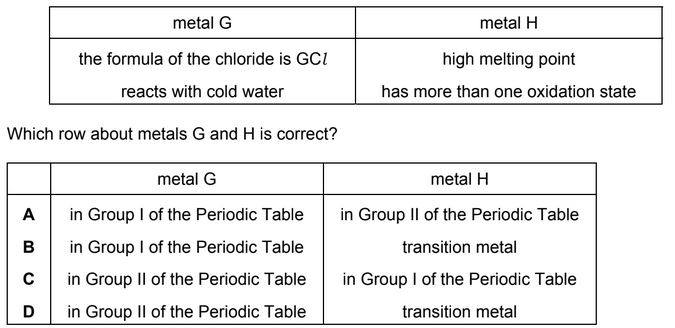
Answer/Explanation
Ans: B
Question
The noble gases are in Group VIII of the Periodic Table.
Which statement explains why noble gases are unreactive?
A They all have eight electrons in their outer shells.
B They all have full outer shells.
C They are all gases.
D They are all monoatomic.
Answer/Explanation
Ans: B
Question
Which statement is correct for all metals?
A They conduct electricity when molten.
B They gain electrons when they form ions.
C They have a low density.
D They have a low melting point.
Answer/Explanation
Ans: A
Question
Which statement about the extraction of metals is correct?
A Aluminium is extracted from the ore bauxite by electrolysis.
B Aluminium is extracted from the ore hematite by electrolysis.
C Iron is extracted from the ore bauxite by electrolysis.
D Iron is extracted from the ore hematite by electrolysis.
Answer/Explanation
Ans: A
Question
Aluminium objects do not need protection from corrosion.
Iron objects must be protected from corrosion.
Which statement explains why aluminium resists corrosion?
A Aluminium does not form ions easily.
B Aluminium does not react with water or air.
C Aluminium has a protective oxide layer.
D Aluminium is below iron in the reactivity series.
Answer/Explanation
Ans: C
Question
Which statements about the thermal decomposition of copper(II) nitrate are correct?
1 A brown gas is given off.
2 A gas which relights a glowing splint is given off.
3 The solid residue is an acidic oxide.
A 1 only B 1 and 2 C 1 and 3 D 2 and 3
Answer/Explanation
Ans: B
Question
Covering iron with zinc prevents the iron from rusting even when the zinc is scratched. Covering iron with tin prevents the iron from rusting, but when the tin is scratched the iron underneath starts to rust.
Which statement is correct?
A Both tin and zinc prevent iron from rusting by sacrificial protection.
B Both tin and zinc prevent iron from rusting by stopping water and carbon dioxide reaching the
iron.
C Tin is more reactive than iron and prevents iron from rusting until it is scratched.
D Zinc loses electrons more easily than iron and prevents iron from rusting by corroding first.
Answer/Explanation
Ans: D
Question
Which statements about the Haber process are correct?
1 One of the raw materials is extracted from liquid air by fractional distillation.
2 One of the raw materials is produced by the reaction of steam and methane.
3 The catalyst for the Haber process is vanadium(V) oxide.
A 1 only B 1 and 2 only C 2 and 3 only D 1, 2 and 3
Answer/Explanation
Ans: B
Question
Which raw material is used in the Contact process?
A air
B ammonia
C carbon
D nitrogen
Answer/Explanation
Ans: A
Question
Lime (calcium oxide) is used to treat waste water from a factory.
Which substance is removed by the lime?
A ammonia
B sodium chloride
C sodium hydroxide
D sulfuric acid
Answer/Explanation
Ans: D
Question
. An alkane molecule of molecular formula \(C_8H_{18}\) undergoes cracking. The equation for the
reaction is shown.
\(C_8H_{18} → Q + 2R\)
Substance R has two carbon atoms per molecule and decolourises aqueous bromine.
What is substance Q?
A butane
B butene
C ethane
D ethene
Answer/Explanation
Ans: A
Question
Fuel X produces carbon dioxide and water when it is burned in air. So does fuel Y.
What could X and Y be?

Answer/Explanation
Ans: D
Question
Which molecule contains only single covalent bonds?
A propane
B propanoic acid
C propene
D propyl propanoate
Answer/Explanation
Ans: A
Question
Alkanes react with chlorine to form chloroalkanes.
Which statement about the reactions of alkanes with chlorine is correct?
A Alkanes react with chlorine by addition.
B The gaseous product turns red litmus blue.
C The chlorine atom in chloroethane is covalently bonded.
D The general formula of the chloroalkanes is \(C_nH_{2n}Cl\).
Answer/Explanation
Ans: C
Question
Part of the structure of a very large molecule is shown.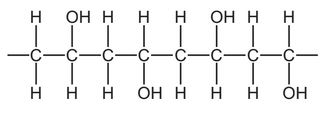
Which term describes the small unit used to make this molecule?
A hydrocarbon
B monomer
C polymer
D saturated
Answer/Explanation
Ans: B
Question
Propene reacts with steam to form propanol.
\(C_3H_6(g) + H_2O(g) → C_3H_7OH(g)\)
Which type of reaction takes place?
A addition
B condensation
C oxidation
D substitution
Answer/Explanation
Ans: A
Question
Which statement about aqueous ethanoic acid is correct?
A It reacts with magnesium to produce a salt and hydrogen.
B It reacts with sodium hydroxide to produce a salt and hydrogen.
C It reacts with ammonium salts to produce ammonia.
D It turns red litmus blue.
Answer/Explanation
Ans: A
Question
The diagram shows the partial structure of Terylene.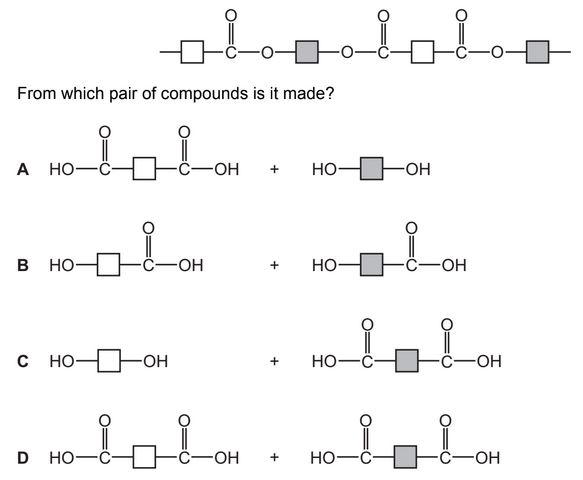
Answer/Explanation
Ans: A
