Question
A gas is placed in a sealed container. The gas has a pressure of one atmosphere and a temperature of \(50^{\circ} \mathrm{C}\).
It is heated to \(100^{\circ} \mathrm{C}\).
Which row describes the cause of the pressure of the gas and the effect of increasing the temperature of the gas?
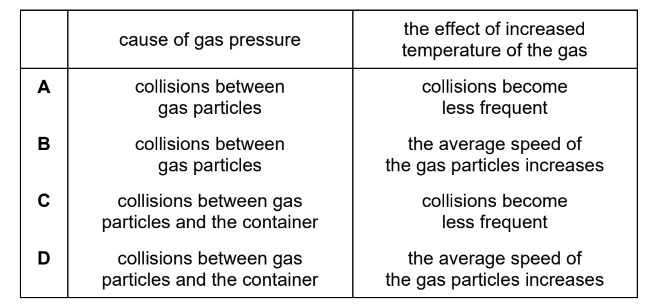
▶️Answer/Explanation
Ans:D
Question
Four experiments, each containing a different acidic gas, are set up as shown.
The dividing glass plates are removed at the same time.
In which set of apparatus does the litmus turn red first?
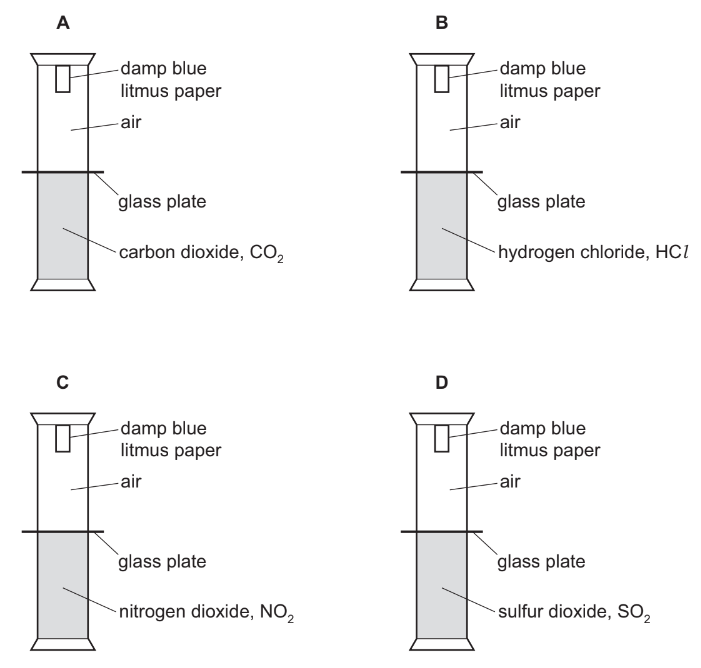
▶️Answer/Explanation
Ans:B
Question
The Group I element potassium forms an ionic bond with the Group VII element fluorine.
Which two ions are produced?
A \(\mathrm{K}^{+}\)and \(\mathrm{F}^{+}\)
B \(\mathrm{K}^{+}\)and \(\mathrm{F}^{-}\)
C \(\mathrm{K}^{-}\)and \(\mathrm{F}^{-}\)
D \(\mathrm{K}^{-}\)and \(\mathrm{F}^{+}\)
▶️Answer/Explanation
Ans:B
Question
\(\mathrm{X}\) and \(\mathrm{Y}\) are atoms.
- \(X\) and \(Y\) have the same number of electron shells.
- \(X\) and \(Y\) have the same number of outer electrons.
- \(\mathrm{X}\) and \(\mathrm{Y}\) have different mass numbers.
Which statements about \(\mathrm{X}\) and \(\mathrm{Y}\) are correct?
\(1 \mathrm{X}\) and \(\mathrm{Y}\) are isotopes.
\(2 \mathrm{X}\) and \(\mathrm{Y}\) have the same total number of electrons.
\(3 \mathrm{X}\) and \(\mathrm{Y}\) have the same chemical properties.
A 1,2 and 3
B 1 and 2 only
C 1 and 3 only
D 2 and 3 only
▶️Answer/Explanation
Ans:A
Question
Lithium chloride is an ionic compound and silicon(IV) oxide is a covalent compound.
Which statement about both compounds is correct?
A They are not soluble in water.
B They conduct electricity when melted.
C They do not conduct electricity in solid form.
D They have low melting points.
▶️Answer/Explanation
Ans:C
Question
Which equations are balanced?
\(1 \mathrm{Fe}_2 \mathrm{O}_3+3 \mathrm{CO} \rightarrow 2 \mathrm{Fe}+3 \mathrm{CO}_2\)
\(2 \mathrm{ZnCO}_3+2 \mathrm{HCl} \rightarrow \mathrm{ZnCl}_2+\mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}\)
\(3 \mathrm{Mg}\left(\mathrm{NO}_3\right)_2+\mathrm{NaOH} \rightarrow \mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{NaNO}_3\)
\(4 \mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CaSO}_4+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:B
Question
Which row shows the formulae of sodium carbonate, zinc nitrate and ammonium sulfate?
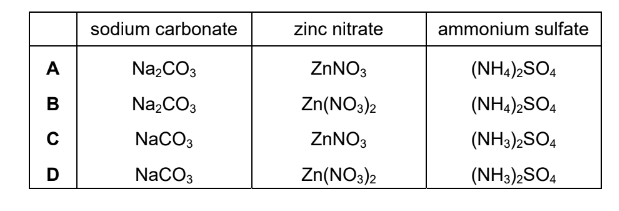
▶️Answer/Explanation
Ans:B
Question
Which statements about hydrogen and oxygen are correct?
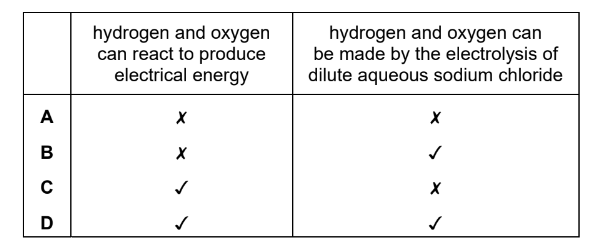
▶️Answer/Explanation
Ans:D
Question
Graphite has a giant covalent structure.
Which statements about graphite are correct?
1 Carbon atoms form four covalent bonds with neighbouring atoms.
2 There are delocalised electrons between layers of carbon atoms.
3 Graphite is a useful lubricant.
4 Graphite is a good conductor of electricity.
A 1 and 2
B 1, 3 and 4
C 2,3 and 4
D 3 and 4 only
▶️Answer/Explanation
Ans:C
Question
Which reaction pathway diagram represents an endothermic reaction?
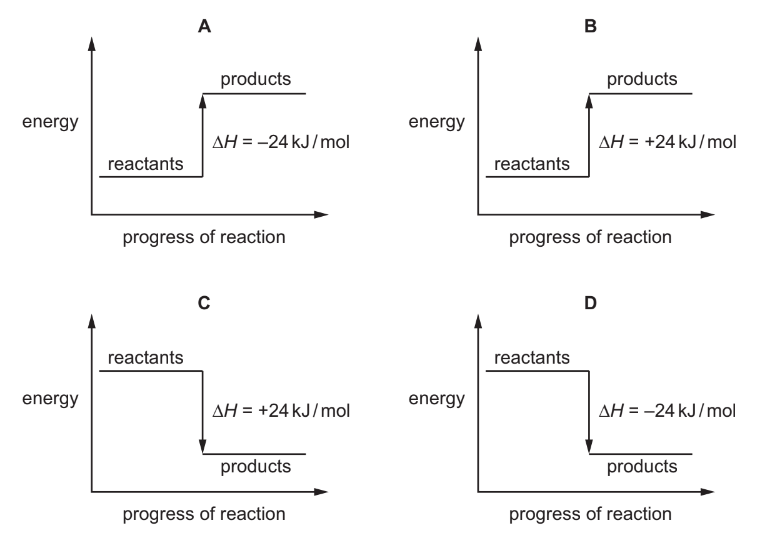
▶️Answer/Explanation
Ans:B
Question
Hydrogen burns in oxygen.
The equation for the reaction is shown.
$
2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}
$
The table shows the bond energies involved.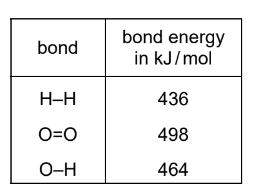
What is the energy given out during the reaction?
A \(-3226 \mathrm{~kJ} / \mathrm{mol}\)
B \(-884 \mathrm{~kJ} / \mathrm{mol}\)
C \(-486 \mathrm{~kJ} / \mathrm{mol}\)
D \(\quad-442 \mathrm{~kJ} / \mathrm{mol}\)
▶️Answer/Explanation
Ans:C
Question
Which process involves a chemical change?
A adding sodium to water
B boiling water
C dissolving sodium chloride in water
D producing water from aqueous sodium chloride
▶️Answer/Explanation
Ans:A
Question
An experiment is carried out to find the rate of reaction between hydrochloric acid and zinc.
$
\mathrm{Zn}(\mathrm{s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{ZnCl}_2(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})
$
The results of the experiment are shown.
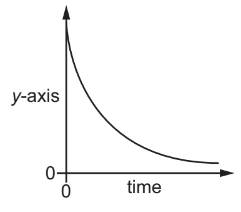
What is the label on the \(y\)-axis?
A amount of \(\mathrm{ZnCl}_2\) produced
B concentration of \(\mathrm{HCl}\)
C mass of \(\mathrm{Zn}\) reacted
D volume of \(\mathrm{H}_2\) produced
▶️Answer/Explanation
Ans:B
Question
Hydrogen peroxide, \(\mathrm{H}_2 \mathrm{O}_2\), decomposes to form water and oxygen.
$
2 \mathrm{H}_2 \mathrm{O}_2(\mathrm{aq}) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g})
$
Manganese(IV) oxide catalyses the decomposition reaction.
The reaction is investigated in four experiments.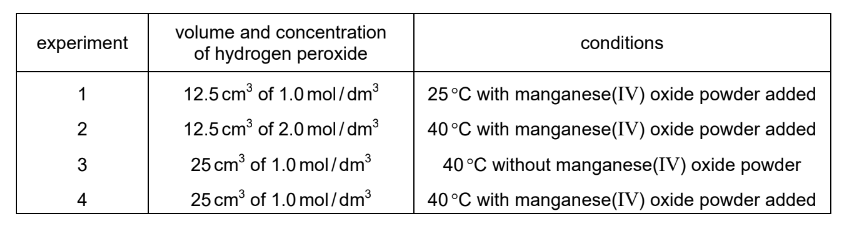
All reactions go to completion and all measurements of gas volumes are at room temperature and pressure.
Which statement is correct?
A Experiment 1 produces less gas than experiment 4, but at the same rate.
B Experiment 2 produces more gas than experiment 1, but at the same rate.
C Experiment 2 and experiment 4 each produce the same volume of gas, but at different rates.
D Experiment 3 and experiment 4 each produce the same volume of gas and at the same rate.
▶️Answer/Explanation
Ans:C
Question
Sulfuric acid is produced by the Contact process.
Which row shows the typical conditions used in the process?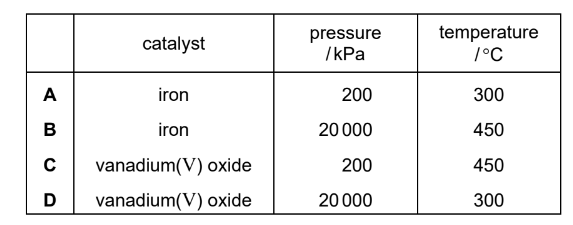
▶️Answer/Explanation
Ans:C
Question
Which equation shows the reduction of copper?
A \(\mathrm{CuO}+\mathrm{C} \rightarrow \mathrm{Cu}+\mathrm{CO}\)
B \(\quad 2 \mathrm{CuS}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CuO}+2 \mathrm{SO}_2\)
C \(\mathrm{Cu}(\mathrm{g}) \rightarrow \mathrm{Cu}(\mathrm{l})\)
D \(\mathrm{Cu}(\mathrm{l}) \rightarrow \mathrm{Cu}(\mathrm{s})\)
▶️Answer/Explanation
Ans:A
Question
Which statement about acids is correct?
A A weak acid partially dissociates in aqueous solution.
B An acid accepts protons when added to water.
C Ethanoic acid acts as a strong acid when added to water.
D Hydrochloric acid is a strong acid that ionises in water to form \(\mathrm{H}^{-}\)ions
▶️Answer/Explanation
Ans:A
Question
Copper(II) sulfate is formed by reacting excess solid copper(II) carbonate with dilute sulfuric acid.
Which processes are part of the preparation of solid copper(II) sulfate?
1 crystallisation
2 distillation
3 filtration
4 titration
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans:A
Question
Which type of reaction is represented by the equation shown?
$
\mathrm{Pb}^{2+}(\mathrm{aq})+2 \mathrm{NO}_3^{-}(\mathrm{aq})+2 \mathrm{Na}^{+}(\mathrm{aq})+2 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow \mathrm{PbI}_2(\mathrm{~s})+2 \mathrm{Na}^{+}(\mathrm{aq})+2 \mathrm{NO}_3{ }^{-}(\mathrm{aq})
$
A addition
B redox
C neutralisation
D precipitation
▶️Answer/Explanation
Ans:D
Question
Which compound is likely to be coloured?
A \(\mathrm{KMnO}_4\)
B \(\mathrm{KNO}_3\)
C \(\mathrm{K}_2 \mathrm{CO}_3\)
D \(\mathrm{K}_2 \mathrm{SO}_4\)
▶️Answer/Explanation
Ans:A
Question
Which statements about the metal zinc are correct?
1 It is extracted from the ore bauxite.
2 It is used to galvanise steel.
3 It is used to make the alloy brass.
4 It reacts with dilute hydrochloric acid to produce hydrogen gas.
A 1,2 and 4
B 1,3 and 4
C 2,3 and 4
D 2 and 3 only
▶️Answer/Explanation
Ans:C
Question
The electronic configurations of four elements, P, Q, R and S, are shown.
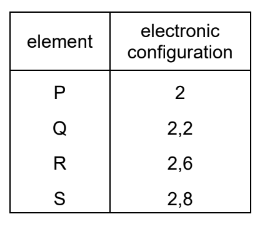
Which elements are unreactive monatomic gases?
A P and Q
B \(\mathrm{P}\) and \(\mathrm{S}\)
C \(Q\) and \(R\)
D S only
▶️Answer/Explanation
Ans:B
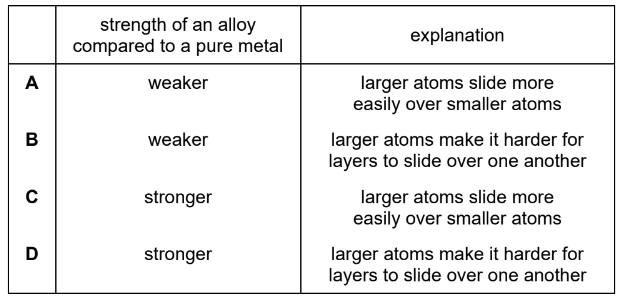
Question
Which row compares the strength of alloys with pure metals and explains the difference in strength?
▶️Answer/Explanation
Ans:D
Question
Zinc oxide reacts with carbon to produce zinc.
Which equation represents this reaction?
A \(2 \mathrm{ZnO}+\mathrm{C} \rightarrow 2 \mathrm{Zn}+\mathrm{CO}\)
B \(2 \mathrm{ZnO}+2 \mathrm{C} \rightarrow 2 \mathrm{Zn}+2 \mathrm{CO}_2\)
C \(\mathrm{ZnO}+\mathrm{C} \rightarrow \mathrm{Zn}+\mathrm{CO}\)
D \(\mathrm{ZnO}+2 \mathrm{C} \rightarrow \mathrm{Zn}+2 \mathrm{CO}_2\)
▶️Answer/Explanation
Ans:C
Question
When a piece of aluminium foil is added to dilute hydrochloric acid, no effervescence is seen.
Which statement explains why no effervescence is seen?
A Aluminium does not make a gas when it reacts with an acid.
B Aluminium has a surface layer of aluminium oxide.
C Aluminium is less reactive than hydrogen.
D Aluminium only reacts with concentrated acid.
▶️Answer/Explanation
Ans:B
Question
Iron nails are stored in an airtight container.
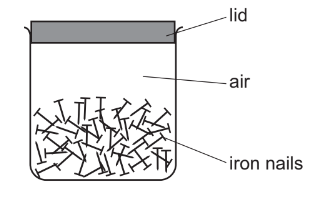
The nails begin to rust after a few days.
How can the rusting of the nails be prevented?
A Leave the lid off.
B Replace the air with argon.
C Put the container in a warm place.
D Seal the container in a bag.
▶️Answer/Explanation
Ans:B
Question
Four substances present in the blast furnace during iron extraction are listed.
1 calcium carbonate
2 carbon dioxide
3 carbon monoxide
4 iron(III) oxide
Which substances are both a reactant and a product during the reactions occurring in the blast furnace?
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
▶️Answer/Explanation
Ans:C
Question
Aluminium is extracted from purified bauxite by electrolysis.
Which row shows the ionic half-equations for the reaction at each electrode?
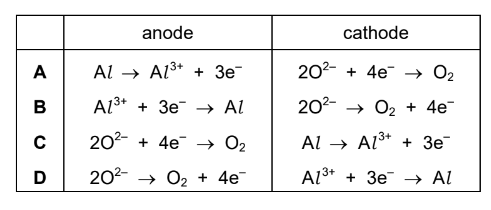
▶️Answer/Explanation
Ans:D
Question
Which test is used to show that a sample of water is pure?
A Evaporate the water to see if any solids remain.
B Heat the water to check its boiling point.
C Test with anhydrous cobalt(II) chloride.
D Use universal indicator paper to check its \(\mathrm{pH}\).
▶️Answer/Explanation
Ans:B
Question
Catalytic converters in car exhausts change polluting gases into non-polluting gases.
Which statements about oxides of nitrogen and car engines are correct?
1 The nitrogen in oxides of nitrogen comes from compounds in gasoline.
2 The oxygen in oxides of nitrogen comes from the air in the car engine.
3 Catalytic converters convert oxides of nitrogen into nitrogen.
A 1 and 2
B 2 and 3
C 2 only
D 3 only
▶️Answer/Explanation
Ans:B
Question
The structures of two molecules, \(X\) and \(Y\), are shown.
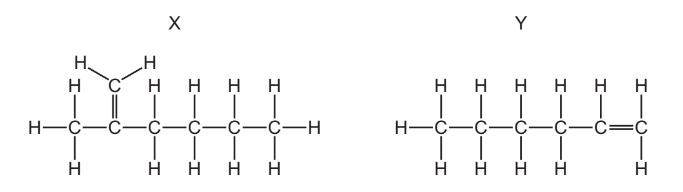
Which row describes X and Y?
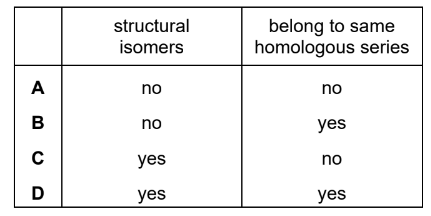
▶️Answer/Explanation
Ans:B
Question
What is the structure of butanoic acid?
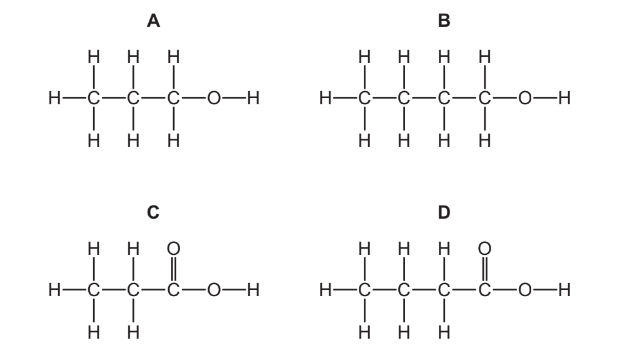
▶️Answer/Explanation
Ans:D
Question
When a mixture of methane and chlorine is exposed to ultraviolet light, a reaction takes place.
Which statements about this reaction are correct?
1 It is an addition reaction.
2 The ultraviolet light provides the activation energy.
3 An equation for the reaction is \(\mathrm{CH}_4+\mathrm{Cl}_2 \rightarrow \mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{H}_2\).
\(4 \mathrm{CH}_3 \mathrm{Cl}\) is made in the reaction.
A 1 and 3
B 1 and 4
C 2 and 3
D 2 and 4
▶️Answer/Explanation
Ans:D
Question
Esters are formed when a carboxylic acid reacts with an alcohol.
What is the catalyst for this reaction?
A aqueous potassium manganate(VII)
B iron
C sulfuric acid
D vanadium(V) oxide
▶️Answer/Explanation
Ans:C
Question
The diagram shows part of a polymer.
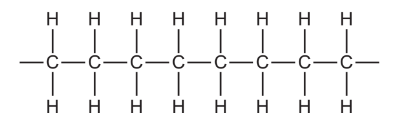
Which diagram shows the monomer from which this polymer is made?
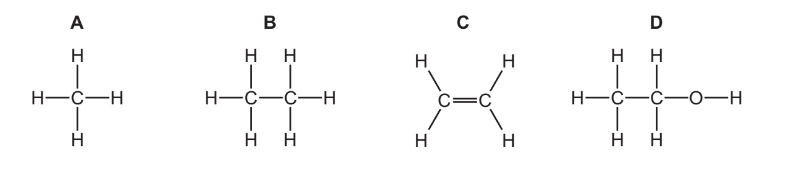
▶️Answer/Explanation
Ans:C
Question
Nylon and PET are polymers.
Which statements about these polymers are correct?
1 They are both condensation polymers.
\(2 \mathrm{HOCH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}\) could be a monomer for both polymers.
3 The complete combustion of both polymers gives two products only.
A 1 and 2
B 1 and 3
C 1 only
D 2 and 3
▶️Answer/Explanation
Ans:C
Question
Ethane is used as a fuel.
Which equation shows the complete combustion of ethane?
A \(2 \mathrm{C}_2 \mathrm{H}_6+7 \mathrm{O}_2 \rightarrow 4 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}\)
B \(2 \mathrm{C}_2 \mathrm{H}_6+5 \mathrm{O}_2 \rightarrow 4 \mathrm{CO}+6 \mathrm{H}_2 \mathrm{O}\)
C \(\mathrm{C}_2 \mathrm{H}_4+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}\)
D \(\mathrm{C}_2 \mathrm{H}_4+2 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}+2 \mathrm{H}_2 \mathrm{O}\)
▶️Answer/Explanation
Ans:A
Question
The painkiller paracetamol is synthesised from 4-aminophenol.
Chromatography is done on an impure sample of paracetamol. The results are shown. The
diagram is not drawn to scale.
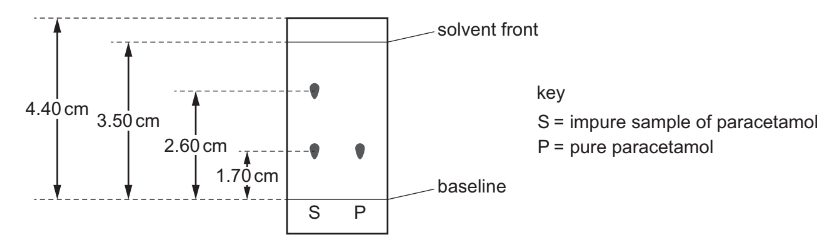
The sample of paracetamol is contaminated with 4-aminophenol only.
What is the \(R_{\mathrm{f}}\) value of 4-aminophenol?
A 0.49
B 0.65
C \(\quad 0.74\)
D 1.35
▶️Answer/Explanation
Ans:C
Question
The equation for the reaction of aqueous calcium nitrate and aqueous sodium hydroxide is shown.
$
\mathrm{Ca}\left(\mathrm{NO}_3\right)_2(\mathrm{aq})+2 \mathrm{NaOH}(\mathrm{aq}) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(\mathrm{~s})+2 \mathrm{NaNO}_3(\mathrm{aq})
$
Which process is used to remove calcium hydroxide from the mixture?
A chromatography
B crystallisation
C distillation
D filtration
▶️Answer/Explanation
Ans:D
Question
The results of two tests on aqueous compound X are given.

What is \(X\) ?
A iron(III) nitrate
B iron(II) nitrate
C iron(III) sulfate
D iron(II) sulfate
▶️Answer/Explanation
Ans:A
