Topic – 1.1
Oxygen melts at –219°C and boils at –183°C.
At which temperature is oxygen a liquid?
A) –225°C
B) –189°C
C) –175°C
D) 25°C
▶️ Answer/Explanation
Ans: B
To determine when oxygen is a liquid, we need to find a temperature between its melting point (–219°C) and boiling point (–183°C).
Let’s evaluate each option:
A) –225°C: Below melting point → solid state
B) –189°C: Between –219°C and –183°C → liquid state (correct answer)
C) –175°C: Above boiling point → gaseous state
D) 25°C: Far above boiling point → gaseous state
Therefore, the only temperature where oxygen exists as a liquid is –189°C.
Topic – 1.1
The pressure of a sample of gas is decreased. The temperature is kept constant.
Which row describes the effects on the particles?
| movement of particles | collisions between particles | |
|---|---|---|
| A | slower | occur less often |
| B | slower | occur with more force |
| C | no change in speed | occur less often |
| D | no change in speed | occur with more force |
▶️ Answer/Explanation
Ans: C
When the pressure of a gas decreases at constant temperature:
1. Particle speed: The temperature remains constant, so the average kinetic energy of the particles doesn’t change. This means their speed stays the same.
2. Collision frequency: Lower pressure means the gas expands, so particles are farther apart. This results in fewer collisions because they have more space to move between collisions.
Looking at the options:
A & B: Incorrect because particle speed doesn’t change (temperature is constant)
D: Incorrect because collisions don’t occur with more force (temperature is constant)
C: Correct – speed remains the same and collisions occur less often
Topic – 2.3
Rubidium has two isotopes, \( _{37}^{85}\textrm{Rb}\) and \( _{37}^{87}\textrm{Rb}\).
Which statement explains why both isotopes have the same chemical properties?
A) They have the same number of protons.
B) They have the same electronic configuration.
C) They have different numbers of neutrons.
D) They have different mass numbers.
▶️ Answer/Explanation
Ans: B
Chemical properties are determined by the arrangement of electrons, which governs how atoms interact with other atoms.
Let’s analyze each option:
A: While true that they have the same protons (37), this alone doesn’t explain chemical properties directly.
B: Correct answer. Isotopes have identical electronic configurations because they have the same number of electrons (equal to protons in neutral atoms), which determines chemical behavior.
C & D: These describe differences between isotopes (neutron number and mass number), but these don’t affect chemical properties.
Key point: Chemical reactions involve electrons, and since isotopes have identical electron arrangements, they behave the same chemically despite different masses.
Topic – 2.4
Which pair of elements react to form a compound with a strong attraction between oppositely charged ions?
A) carbon and bromine
B) carbon and nitrogen
C) sodium and oxygen
D) sodium and potassium
▶️ Answer/Explanation
Ans: C
We need to identify which pair forms an ionic compound with strong electrostatic attraction.
Let’s evaluate each option:
A) C + Br: Forms covalent bonds (both non-metals)
B) C + N: Forms covalent bonds (both non-metals)
C) Na + O: Forms ionic compound Na₂O (metal + non-metal). Sodium donates electrons to oxygen, creating Na⁺ and O²⁻ ions with strong attraction.
D) Na + K: Both are metals and don’t form ionic compounds with each other.
Key points:
– Ionic compounds form between metals and non-metals
– Sodium (Group I) easily loses electrons
– Oxygen (Group VI) readily gains electrons
This makes sodium oxide (Na₂O) a classic ionic compound with strong ionic bonds.
Topic – 2.7
Four substances, P, Q, R and S, are described.
- P is diatomic.
- Q is a good conductor of electricity when solid and when molten.
- R is a silver solid with a very high melting point.
- S reacts with oxygen to form a brown gas.
Which substances are metals?
A) P and Q
B) P and S
C) Q and R
D) R and S
▶️ Answer/Explanation
Ans: C
Let’s analyze each substance’s properties to identify metals:
P (diatomic): Most metals aren’t diatomic (except some like Hg in vapor). This suggests a non-metal (like O₂, N₂).
Q (conducts electricity when solid/molten): This is characteristic of metals (metallic bonding with delocalized electrons).
R (silver solid, high melting point): Typical metal properties (silvery luster, high m.p. due to strong metallic bonds).
S (forms brown gas with O₂): Likely a non-metal (e.g., nitrogen forms brown NO₂).
Therefore:
– Q and R show metallic properties
– P and S are non-metals
Metals are characterized by:
– Electrical conductivity
– Malleability/ductility
– Shiny luster
– High melting points (usually)
Topic – 2.5
Which diagram shows the covalent bonding in a molecule of carbon dioxide?
A) O–C–O
B) O=C–O
C) O=C=O
D) O≡C≡O
▶️ Answer/Explanation
Ans: C
Carbon dioxide (CO₂) has a linear molecular geometry with double bonds between the carbon atom and each oxygen atom. Each oxygen shares two pairs of electrons with the central carbon atom, forming two double bonds (O=C=O).
Option A shows single bonds which is incorrect. Option B shows one double and one single bond which doesn’t represent CO₂’s symmetrical structure. Option D shows a triple bond which doesn’t occur in CO₂.
Topic – 2.5
The bonding, structure and melting point of sodium chloride and sulfur dichloride are shown.
| compound | bonding | structure | melting point / °C |
|---|---|---|---|
| sodium chloride | ionic | giant lattice | 801 |
| sulfur dichloride | covalent | simple molecular | -121 |
Why does sulfur dichloride have a lower melting point than sodium chloride?
A) The covalent bonds in sulfur dichloride are weaker than the attractive forces between molecules in sodium chloride.
B) The covalent bonds in sulfur dichloride are weaker than the ionic bonds in sodium chloride.
C) The attractive forces between molecules in sulfur dichloride are weaker than the attractive forces between molecules in sodium chloride.
D) The attractive forces between molecules in sulfur dichloride are weaker than the ionic bonds in sodium chloride.
▶️ Answer/Explanation
Ans: D
Sulfur dichloride (SCl₂) is a simple covalent molecule with weak intermolecular forces (van der Waals forces) between molecules, while sodium chloride (NaCl) has a giant ionic lattice structure with strong electrostatic forces between ions.
The key difference is that melting NaCl requires breaking strong ionic bonds, while melting SCl₂ only requires overcoming weak intermolecular forces. The covalent bonds within SCl₂ molecules aren’t broken during melting, making options A and B incorrect. Option C is wrong because NaCl doesn’t have molecules with intermolecular forces – it has an ionic lattice.
Topic – 2.6
Diamond and graphite have giant covalent structures of carbon atoms.
Which statement describes graphite?
A) It has a strong, rigid three-dimensional structure.
B) It has four strong covalent bonds between each carbon atom.
C) It has layers, which can slide over each other.
D) It has no delocalised electrons so does not conduct electricity.
▶️ Answer/Explanation
Ans: C
Graphite has a layered structure where each carbon atom forms three covalent bonds, creating hexagonal sheets. These layers can slide over each other, which is why graphite is slippery and used in pencils.
Option A describes diamond’s structure. Option B is incorrect because graphite has only three bonds per carbon atom (not four). Option D is wrong because graphite does have delocalized electrons between layers, which allows it to conduct electricity.
Topic – 2.7
Which row explains the malleability and electrical conductivity of a solid metal?
| malleability | electrical conductivity | |
|---|---|---|
| A | Delocalised electrons can move freely through the structure. | Delocalised electrons can move freely through the structure. |
| B | Delocalised electrons can move freely through the structure. | Positive ions can move freely through the structure. |
| C | Rows of positive ions can slide over each other. | Delocalised electrons can move freely through the structure. |
| D | Rows of positive ions can slide over each other. | Positive ions can move freely through the structure. |
▶️ Answer/Explanation
Ans: C
Metals are malleable because their positive ions are arranged in layers that can slide over each other when force is applied, while the sea of delocalized electrons maintains the metallic bonding.
Electrical conductivity in metals is due to the movement of delocalized electrons (not positive ions, making options B and D incorrect). Option A incorrectly attributes malleability to electron movement rather than ion layer movement.
Topic – 3.3
The equation for the decomposition of ammonium carbonate, \((\text{NH}_4)_2\text{CO}_3\), is shown.
\[(\text{NH}_4)_2\text{CO}_3(s) \rightarrow 2\text{NH}_3(g) + \text{CO}_2(g) + \text{H}_2\text{O}(l)\]
[\(M: (\text{NH}_4)_2\text{CO}_3, 96\)]
The total volume of gas produced is 360 cm\(^3\) at r.t.p.
Which mass of ammonium carbonate, \((\text{NH}_4)_2\text{CO}_3\), is decomposed?
A) 0.24 g
B) 0.48 g
C) 0.96 g
D) 1.44 g
▶️ Answer/Explanation
Ans: B
1. First calculate total moles of gas produced: 360 cm³ = 0.36 dm³. At r.t.p., 1 mole occupies 24 dm³, so moles of gas = 0.36/24 = 0.015 mol.
2. From the equation: 1 mole \((\text{NH}_4)_2\text{CO}_3\) produces 3 moles of gas (2 NH₃ + 1 CO₂).
3. So moles of \((\text{NH}_4)_2\text{CO}_3\) decomposed = 0.015/3 = 0.005 mol.
4. Mass = moles × Mr = 0.005 × 96 = 0.48 g.
Note: H₂O is liquid and doesn’t contribute to gas volume at r.t.p.
Topic – 3.1
What is the empirical formula of a compound that contains 3.66 g of hydrogen, 37.8 g of phosphorus and 58.5 g of oxygen?
A) H6P2O6
B) H4PO4
C) H3PO3
D) HPO
▶️ Answer/Explanation
Ans: C
To find the empirical formula:
1. Convert masses to moles:
- Hydrogen: 3.66 g ÷ 1 g/mol = 3.66 mol
- Phosphorus: 37.8 g ÷ 31 g/mol ≈ 1.22 mol
- Oxygen: 58.5 g ÷ 16 g/mol ≈ 3.66 mol
2. Divide by smallest number of moles (1.22):
- H: 3.66 ÷ 1.22 ≈ 3
- P: 1.22 ÷ 1.22 = 1
- O: 3.66 ÷ 1.22 ≈ 3
3. This gives the ratio H3PO3, which matches option C.
Topic – 4.1
Aqueous copper(II) sulfate is electrolysed using graphite electrodes.
Which row identifies the product and observations at each electrode during the electrolysis?
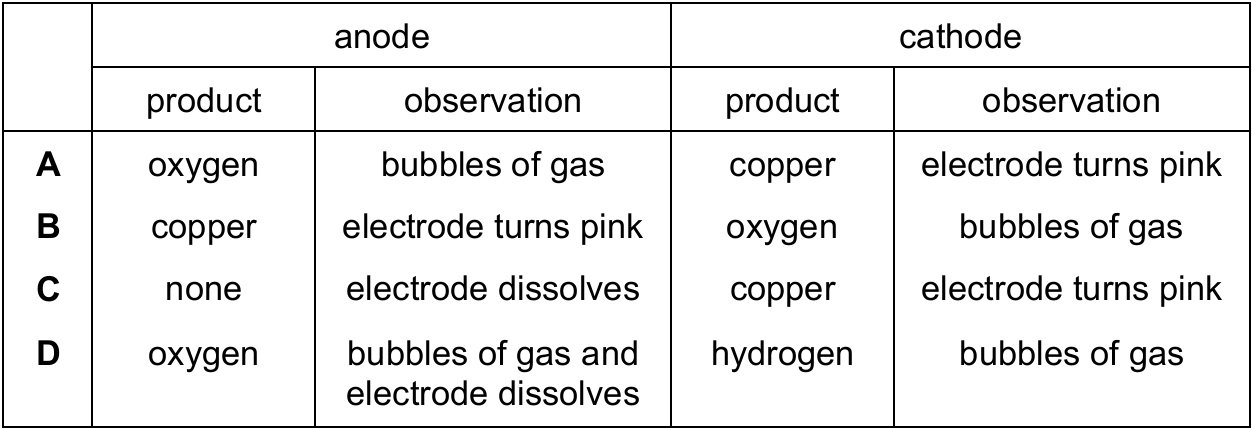
▶️ Answer/Explanation
Ans: A
In the electrolysis of aqueous copper(II) sulfate:
At the anode (positive electrode):
- OH– ions are discharged, producing oxygen gas (bubbles observed)
- 4OH– → O2 + 2H2O + 4e–
At the cathode (negative electrode):
- Cu2+ ions are reduced to copper metal, which deposits on the electrode (pink/brown color observed)
- Cu2+ + 2e– → Cu
Graphite electrodes are inert and don’t dissolve. This matches option A.
Topic – 4.1
Molten sodium chloride is electrolysed using inert electrodes.
Which row shows the products formed at the cathode and anode?
| cathode | anode | |
|---|---|---|
| A | chlorine | hydrogen |
| B | chlorine | sodium |
| C | hydrogen | chlorine |
| D | sodium | chlorine |
▶️ Answer/Explanation
Ans: D
In the electrolysis of molten sodium chloride:
At the cathode (negative electrode):
- Na+ ions are reduced to sodium metal: Na+ + e– → Na
At the anode (positive electrode):
- Cl– ions are oxidized to chlorine gas: 2Cl– → Cl2 + 2e–
This matches option D. Note that in aqueous solution, hydrogen would form at the cathode instead, but in molten NaCl, sodium metal is produced.
Topic – 5.1
The equation for the formation of ammonia is shown.
N2 + 3H2 → 2NH3
The reaction pathway diagram for the reaction is shown.

What is the enthalpy change for the reaction?
A) −592 kJ/mol
B) −92 kJ/mol
C) +92 kJ/mol
D) +592 kJ/mol
▶️ Answer/Explanation
Ans: B
The enthalpy change (ΔH) is the difference between the products and reactants:
From the diagram, the total energy released is -342 kJ/mol, which represents the energy difference between the activated complex and the products.
The activation energy is +250 kJ/mol (energy difference between reactants and activated complex).
Therefore, the enthalpy change is:
ΔH = Energy of products – Energy of reactants
We can calculate it as: ΔH = Activation energy – Total energy released
ΔH = 250 kJ/mol – 342 kJ/mol = -92 kJ/mol
This matches option B.
Topic – 6.2
Sulfur dioxide is converted to sulfur trioxide in the Contact process.
The conditions used are 450 °C and 200 kPa with a vanadium(V) oxide catalyst.
Which row describes and explains the effect of changing conditions on the rate of reaction?
| change in conditions | effect on rate | explanation | |
|---|---|---|---|
| A | no catalyst | lower | the activation energy is higher |
| B | higher pressure | higher | the particles have more kinetic energy |
| C | lower temperature | lower | the particles collide more frequently |
| D | lower pressure | higher | there are more particles per unit volume |
▶️ Answer/Explanation
Ans: A
Analyzing each option:
A) Correct – Without a catalyst, the activation energy would be higher, resulting in a lower rate of reaction.
B) Incorrect – While higher pressure does increase the rate, the explanation is wrong (it’s because there are more frequent collisions, not because particles have more kinetic energy).
C) Incorrect – Lower temperature does decrease the rate, but the explanation is wrong (particles collide less frequently at lower temperatures).
D) Incorrect – Lower pressure decreases the rate (fewer particles per unit volume).
The catalyst provides an alternative reaction pathway with lower activation energy, so removing it would increase the activation energy and decrease the rate.
Topic – 6.3
Hydrogen gas reacts with iodine gas to form hydrogen iodide gas in an equilibrium reaction.
\[ H_2(g) + I_2(g) \rightleftharpoons 2HI(g) \quad \Delta H = +26.5 \, kJ/mol \]
Which changes increase the yield of HI at equilibrium?
- adding a catalyst
- adding more hydrogen gas
- increasing the pressure
- increasing the temperature
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: D
1. Adding a catalyst doesn’t affect equilibrium position, only speeds up the reaction.
2. Adding more hydrogen gas (a reactant) shifts equilibrium to the right (Le Chatelier’s principle), increasing HI yield.
3. Increasing pressure has no effect here as there are equal moles of gas on both sides (2 vs 2).
4. Increasing temperature favors the endothermic reaction (forward reaction is endothermic as ΔH is positive), increasing HI yield.
Therefore, only changes 2 and 4 increase HI yield.
Topic – 6.4
The equation for the reaction of carbon with carbon dioxide is shown.
\[ C + CO_2 \rightarrow 2CO \]
Which row identifies the carbon atom that is reduced and its change in oxidation number?
| atom that is reduced | change in oxidation number | |
|---|---|---|
| A | carbon in CO2 | +2 → +4 |
| B | carbon in CO2 | +4 → +2 |
| C | elemental carbon, C | 0 → +2 |
| D | elemental carbon, C | +2 → 0 |
▶️ Answer/Explanation
Ans: B
1. In CO2, carbon has oxidation number +4 (oxygen is -2 each).
2. In CO, carbon has oxidation number +2 (oxygen is -2).
3. Elemental carbon (C) has oxidation number 0.
4. The carbon in CO2 is reduced (oxidation number decreases from +4 to +2).
5. Elemental carbon is oxidized (oxidation number increases from 0 to +2).
Therefore, the correct answer is B, showing the reduction of carbon in CO2 with the correct change in oxidation number.
Topic – 6.4
Aqueous iron(II) sulfate is added to acidified potassium manganate(VII). The purple colour of the potassium manganate(VII) disappears.
Aqueous potassium iodide is added to acidified potassium dichromate(VI). A dark brown solution forms.
Which row identifies the role of the iron(II) sulfate and the potassium dichromate(VI) in these reactions?
| iron(II) sulfate | potassium dichromate(VI) | |
|---|---|---|
| A | oxidising agent | oxidising agent |
| B | oxidising agent | reducing agent |
| C | reducing agent | reducing agent |
| D | reducing agent | oxidising agent |
▶️ Answer/Explanation
Ans: D
1. In the first reaction, iron(II) sulfate causes the purple manganate(VII) to decolorize, meaning Fe2+ is oxidized to Fe3+, so it acts as a reducing agent.
2. In the second reaction, potassium dichromate(VI) oxidizes iodide ions (I–) to iodine (I2, which causes the brown color), so it acts as an oxidizing agent.
3. Therefore, iron(II) sulfate is the reducing agent and potassium dichromate(VI) is the oxidizing agent.
Topic – 7.1
Which row shows the difference between a weak acid and a strong acid?
| weak acid | strong acid | |
|---|---|---|
| A | fully dissociated | partially dissociated |
| B | concentrated | dilute |
| C | dilute | concentrated |
| D | partially dissociated | fully dissociated |
▶️ Answer/Explanation
Ans: D
1. The key difference between weak and strong acids is their degree of dissociation in water.
2. Strong acids completely dissociate into ions in water (e.g., HCl → H+ + Cl–).
3. Weak acids only partially dissociate, establishing an equilibrium between dissociated and undissociated molecules (e.g., CH3COOH ⇌ H+ + CH3COO–).
4. Options B and C confuse strength (degree of dissociation) with concentration (amount of acid per unit volume).
5. Option A reverses the definitions.
Topic – 7.1
Which substance turns methyl orange red?
A) aqueous ammonia
B) dilute hydrochloric acid
C) aqueous sodium hydroxide
D) distilled water
▶️ Answer/Explanation
Ans: B
1. Methyl orange is a pH indicator that turns red in acidic conditions (pH < 3.1).
2. Dilute hydrochloric acid is strongly acidic (pH ~1-2), so it turns methyl orange red.
3. Aqueous ammonia and sodium hydroxide are alkaline and would turn methyl orange yellow.
4. Distilled water is neutral (pH 7) and would give an intermediate orange color with methyl orange.
Topic – 7.2
Which row describes zinc oxide and calcium oxide?
| zinc oxide | calcium oxide | |
|---|---|---|
| A | basic | acidic |
| B | acidic | basic |
| C | amphoteric | acidic |
| D | amphoteric | basic |
▶️ Answer/Explanation
Ans: D
Zinc oxide is amphoteric, meaning it can react with both acids and bases. Calcium oxide is a basic oxide that reacts with acids to form salts and water. Therefore, the correct combination is amphoteric for zinc oxide and basic for calcium oxide.
Topic – 8.4
Which row shows the properties of a transition element?
| catalyst | colour of oxide | electrical conductivity | |
|---|---|---|---|
| A | yes | red | good |
| B | yes | green | poor |
| C | no | yellow | good |
| D | no | white | poor |
▶️ Answer/Explanation
Ans: A
Transition elements typically act as catalysts (e.g., iron in the Haber process), form colored compounds (red oxide in this case), and are good conductors of electricity due to their metallic bonding and delocalized electrons. These are all characteristic properties of transition metals.
Topic – 8.3
Fluorine is the element at the top of Group VII of the Periodic Table.
Which statement describes fluorine?
A) It is inert.
B) It is monatomic.
C) It is non-metallic.
D) It is a solid at room temperature.
▶️ Answer/Explanation
Ans: C
Fluorine is a highly reactive non-metallic element (option C). It is not inert (A is wrong), exists as diatomic F₂ molecules (B is wrong), and is a pale yellow gas at room temperature (D is wrong). As a halogen, fluorine is one of the most reactive elements in the periodic table.
Topic – 9.4
When aluminium is placed in dilute hydrochloric acid, there is no reaction.
When zinc is placed in dilute hydrochloric acid, bubbles of gas are immediately given off.
Which statement correctly explains these observations?
A) Aluminium is coated with a layer of aluminium oxide.
B) Aluminium is more reactive than hydrogen.
C) Aluminium is less reactive than zinc.
D) Zinc is less reactive than hydrogen.
▶️ Answer/Explanation
Ans: A
Aluminium naturally forms a protective oxide layer that prevents further reaction with acids (option A). While aluminium is more reactive than hydrogen (B is true), this doesn’t explain the lack of initial reaction. Zinc is actually less reactive than aluminium (C is wrong), and zinc is more reactive than hydrogen (D is wrong). The key factor is the oxide layer on aluminium that initially prevents reaction.
Topic – 9.5
Which statements about the use of sacrificial protection to prevent iron from rusting are correct?
- A more reactive metal than iron is used as a sacrificial protector because it undergoes reduction before iron.
- Zinc is used as a sacrificial protector because it gains electrons more readily than iron.
- Copper is not used as a sacrificial protector because it is less reactive than iron.
- Magnesium is used as a sacrificial protector because it loses electrons more readily than iron.
A) 1 and 2
B) 1 and 4
C) 2 and 3
D) 3 and 4
▶️ Answer/Explanation
Ans: D
Statement 1 is incorrect because the sacrificial metal undergoes oxidation (loses electrons), not reduction. Statement 2 is wrong because zinc loses electrons more readily, not gains them. Statement 3 is correct – copper is less reactive than iron and wouldn’t protect it. Statement 4 is correct – magnesium is more reactive and loses electrons more readily than iron, making it an excellent sacrificial protector. Therefore, the correct combination is 3 and 4 (option D).
Topic – 9.6
Aluminium is extracted from its ore by electrolysis.
What is the role of cryolite in this process?
A) to lower the operating temperature
B) to lower the boiling point of bauxite
C) to raise the melting point of bauxite
D) to act as a catalyst
▶️ Answer/Explanation
Ans: A
Cryolite (Na3AlF6) is added to the alumina (Al2O3) in the Hall-Héroult process for aluminum extraction. The main purpose of cryolite is to lower the melting point of the alumina from about 2072°C to around 950°C, which significantly reduces the energy required for the electrolysis process. This makes the extraction more economically viable. Cryolite doesn’t act as a catalyst (it’s not regenerated), nor does it affect the boiling point of bauxite. It specifically lowers the operating temperature of the electrolytic cell.
Topic – 10.3
Which row identifies two greenhouse gases and three processes by which they contribute to global warming?
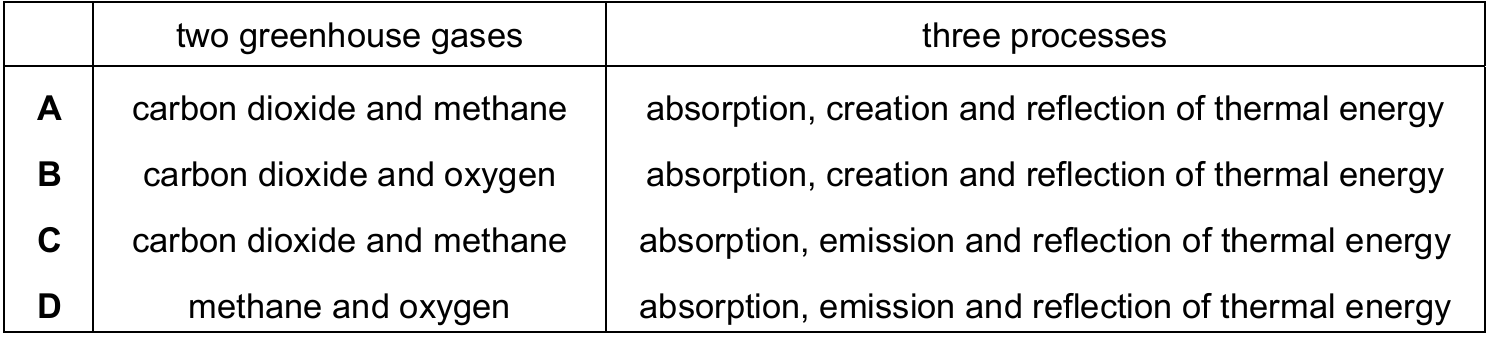
▶️ Answer/Explanation
Ans: C
The two primary greenhouse gases are carbon dioxide (CO2) and methane (CH4). Oxygen (O2) is not a greenhouse gas. Greenhouse gases contribute to global warming through three main processes:
- Absorption of infrared radiation emitted by the Earth’s surface
- Emission of infrared radiation in all directions, including back toward the Earth’s surface
- Reflection of some of this thermal energy
The term “creation” of thermal energy is incorrect in this context, as greenhouse gases don’t create energy but rather trap and redistribute existing thermal energy. Therefore, option C is correct with the right combination of gases and accurate processes.
Topic – 10.2
Which mixture contains all of the elements in a typical NPK fertiliser?
A) ammonium nitrate and calcium phosphate
B) ammonium phosphate and potassium chloride
C) potassium nitrate and ammonium chloride
D) potassium carbonate and ammonium nitrate
▶️ Answer/Explanation
Ans: B
NPK fertilizers contain three essential elements for plant growth:
- N – Nitrogen (found in ammonium compounds)
- P – Phosphorus (found in phosphate compounds)
- K – Potassium (found in potassium compounds)
Analyzing the options:
Option B (ammonium phosphate and potassium chloride) contains all three: – NH4H2PO4 (ammonium phosphate) provides N and P – KCl (potassium chloride) provides K
The other options are missing at least one element: – A lacks potassium – C lacks phosphorus – D lacks phosphorus
Topic – 11.5
Bromine reacts with but-2-ene.
What is the displayed formula of the product of this reaction?
A) 
B) 
C) 
D) 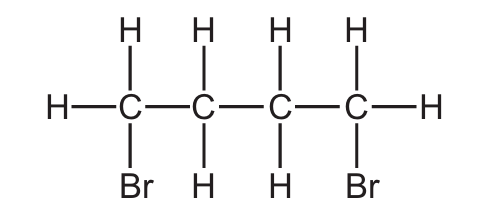
▶️ Answer/Explanation
Ans: C
This is an electrophilic addition reaction of bromine with but-2-ene (CH3CH=CHCH3). The reaction proceeds as follows:
- The π electrons in the C=C double bond attack a bromine molecule, forming a bromonium ion intermediate
- The second bromine ion then attacks from the opposite side (anti-addition)
- The product is 2,3-dibromobutane
The correct structure shows two bromine atoms added to the second and third carbon atoms (the original double bond positions) in an anti configuration. The methyl groups (CH3) remain on the first and fourth carbons. Option C correctly shows this arrangement with the bromine atoms on adjacent carbons in a trans configuration (dashed and wedged bonds indicating they’re on opposite sides).
Topic – 11.3
Which statement is correct?
A) Bitumen is used as a fuel for ships.
B) Coal, natural gas and oxygen are all fuels.
C) Hydrogen is the main constituent of natural gas.
D) Petroleum is separated into useful substances by fractional distillation.
▶️ Answer/Explanation
Ans: D
Let’s analyze each option:
A) Incorrect – Bitumen is too viscous and is primarily used for road surfacing, not as ship fuel.
B) Incorrect – While coal and natural gas are fuels, oxygen is an oxidizer, not a fuel.
C) Incorrect – The main constituent of natural gas is methane (CH4), not hydrogen.
D) Correct – Petroleum (crude oil) is indeed separated into useful fractions (like gasoline, diesel, kerosene) through fractional distillation in oil refineries. This process takes advantage of the different boiling points of the hydrocarbon components.
Fractional distillation works by heating crude oil to vaporize it, then allowing it to condense at different temperatures in a fractionating column, with lighter fractions (lower boiling points) collected at the top and heavier fractions at the bottom.
Topic – 11.4
Which statement explains why ethanoic acid is saturated?
A) The molecule dissociates completely in water.
B) There is a carbon-oxygen double bond in the molecule.
C) The carbon-carbon bond in the molecule is a single bond.
D) All the carbon-hydrogen bonds in the molecule are single bonds.
▶️ Answer/Explanation
Ans: C
A saturated compound is one where all carbon-carbon bonds are single bonds, with no double or triple bonds between carbon atoms. Ethanoic acid (CH₃COOH) has a single bond between its two carbon atoms, making it a saturated compound.
Option A is incorrect because dissociation relates to acidity, not saturation. Option B is incorrect because while there is a C=O bond, saturation refers to carbon-carbon bonds. Option D is incorrect because saturation specifically refers to carbon-carbon bonds, not carbon-hydrogen bonds.
Topic – 11.1
Which statement about compounds in the same homologous series is correct?
A) They have the same chemical properties because they have the same number of carbon atoms.
B) They have the same physical properties because they have the same number of carbon atoms.
C) They have different chemical properties because they have different numbers of carbon atoms.
D) They have different physical properties because they have different numbers of carbon atoms.
▶️ Answer/Explanation
Ans: D
Compounds in a homologous series have similar chemical properties but gradually changing physical properties as the number of carbon atoms increases. The key points are:
1. All members have the same functional group, giving them similar chemical properties (options A and C are incorrect about chemical properties).
2. As chain length increases, physical properties like boiling point change systematically (option B is incorrect about physical properties).
3. Option D correctly states that different numbers of carbon atoms lead to different physical properties while maintaining similar chemical properties.
Topic – 11.4
Which row shows the properties of methane?
| soluble in water | state at room temperature | gives a positive test with aqueous bromine | |
|---|---|---|---|
| A | no | gas | no |
| B | no | gas | yes |
| C | yes | liquid | no |
| D | yes | liquid | yes |
▶️ Answer/Explanation
Ans: A
Methane (CH₄) has the following properties:
1. Solubility: It is not soluble in water because it’s non-polar while water is polar (eliminates options C and D).
2. State: At room temperature, methane is a gas (eliminates options C and D which say it’s liquid).
3. Bromine test: Methane doesn’t react with aqueous bromine because it’s a saturated hydrocarbon (eliminates option B).
Therefore, option A correctly describes all properties of methane.
Topic – 11.6
The table shows two methods used to make ethanol.
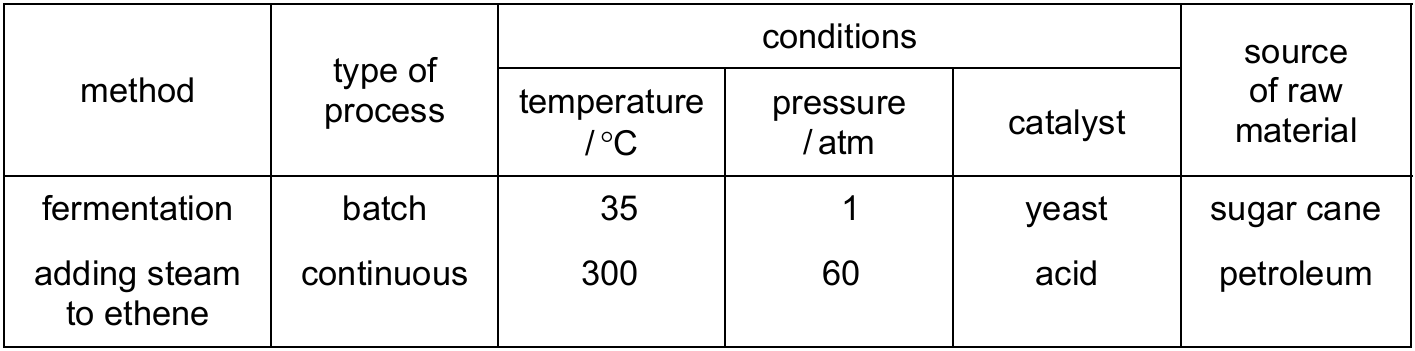
Which statement gives an advantage of preparing ethanol by fermentation rather than by adding steam to ethene?
A) Fermentation takes several days to complete.
B) Little energy is used in the fermentation process.
C) The fermentation of glucose from sugar cane produces pure ethanol.
D) Fermentation uses a non-renewable raw material.
▶️ Answer/Explanation
Ans: B
Comparing the two methods:
1. Fermentation advantages:
– Uses renewable resources (sugar cane) (option D is incorrect)
– Operates at low temperature and pressure (option B is correct)
– Doesn’t require high energy inputs
2. Hydration of ethene disadvantages:
– Requires high temperature and pressure (energy intensive)
– Uses non-renewable petroleum
Option A describes a disadvantage of fermentation. Option C is incorrect as fermentation produces a mixture that needs distillation.
Topic – 11.5
Which equation represents an addition reaction?
A) CH3CHO + HCN → CH3CH(OH)CN
B) C6H6 + Br2 → C6H5Br + HBr
C) NH4Br → NH3 + HBr
D) C14H30 → C2H4 + C8H18 + C4H8
▶️ Answer/Explanation
Ans: A
An addition reaction is where two or more molecules combine to form a single product with no other products.
1. Option A: This shows the addition of HCN to ethanal (CH3CHO) forming a single product (correct answer).
2. Option B: This is a substitution reaction where Br replaces H in benzene.
3. Option C: This is a decomposition reaction where NH4Br breaks down.
4. Option D: This is cracking, a type of decomposition reaction.
Only option A shows two reactants combining to form one product with all atoms incorporated into the final molecule.
Topic – 11.8
The structure of part of a polymer is shown.
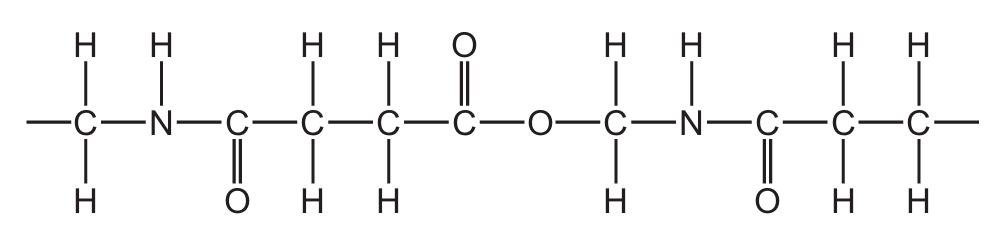
How many amide and ester linkages are included in the structure shown?
| amide linkages | ester linkages | |
|---|---|---|
| A | 1 | 0 |
| B | 1 | 1 |
| C | 2 | 1 |
| D | 2 | 2 |
▶️ Answer/Explanation
Ans: C
To solve this, we need to identify the amide (-CONH-) and ester (-COO-) linkages in the polymer structure:
- Amide linkages are formed between the carbonyl carbon (C=O) and nitrogen (N-H) groups. In the given structure, there are two such connections visible in the repeating unit.
- Ester linkages are formed between the carbonyl carbon (C=O) and oxygen (O) groups. There is one clear ester linkage visible in the repeating unit.
The structure shows a repeating pattern with two amide bonds and one ester bond per unit, making option C (2 amide and 1 ester linkages) the correct choice.
Topic – 11.8
Which structure represents part of a protein?
A) 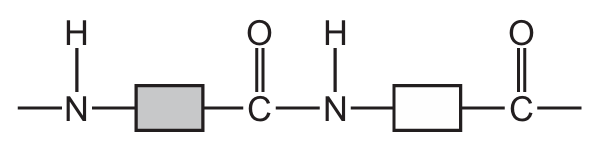
B) 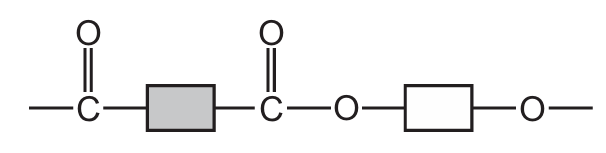
C) 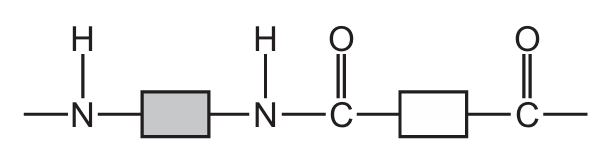
D) 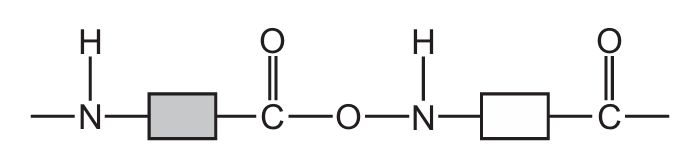
▶️ Answer/Explanation
Ans: A
Proteins are polymers made up of amino acids linked by peptide bonds (a type of amide bond). The key characteristics to look for are:
- The repeating -NH-CH-CO- backbone structure
- Peptide bonds between amino acids (shown as -CONH-)
- Presence of side chains (R groups) attached to the central carbon
Option A shows this characteristic protein structure with clear peptide bonds between amino acid residues. The other options show different types of polymers (B shows ester linkages typical of polyesters, C shows a mix of bonds, and D shows a more complex structure not typical of simple proteins).
Topic – 12.1
Which piece of apparatus can only measure a single fixed volume?
A) a 250 cm3 beaker
B) a 50 cm3 burette
C) a 100 cm3 measuring cylinder
D) a 25 cm3 volumetric pipette
▶️ Answer/Explanation
Ans: D
Let’s analyze each option:
- A) Beaker – Can hold various volumes up to its capacity, not precise for measuring
- B) Burette – Can measure variable volumes with high precision
- C) Measuring cylinder – Can measure different volumes within its range
- D) Volumetric pipette – Designed to measure and transfer a single, precise fixed volume (25 cm3 in this case)
Volumetric pipettes are calibrated to deliver only one specific volume with high accuracy, making them unique among the options for measuring a single fixed volume. This is why they’re commonly used in titrations and other precise analytical work where exact volumes are required.
Topic – 12.4
Pure solid copper(II) nitrate can be obtained from a mixture of copper(II) nitrate and copper powder.
Three stages in the method are listed.
X – add water and stir
Y – crystallise
Z – filter
After the three stages, the copper(II) nitrate is washed and dried.
What is the correct order of stages X, Y and Z to obtain pure solid copper(II) nitrate from the mixture?
A) X → Y → Z
B) X → Z → Y
C) Y → X → Z
D) Z → X → Y
▶️ Answer/Explanation
Ans: B
The correct sequence is:
- X – Add water and stir: Copper(II) nitrate is soluble in water while copper powder is not. Stirring helps dissolve the copper(II) nitrate.
- Z – Filter: This removes the insoluble copper powder, leaving a solution of pure copper(II) nitrate.
- Y – Crystallise: By evaporating some water or cooling the solution, pure copper(II) nitrate crystals form.
Key points:
- You must dissolve first to separate the soluble and insoluble components
- Filtration must come before crystallization to remove impurities
- Crystallization is the final step to obtain the pure solid
This is a standard purification technique for separating a soluble compound from an insoluble one.
Topic – 12.5
Which row describes a test and the observation for aqueous sulfate ions?
| test | observation | |
|---|---|---|
| A | add dilute nitric acid | a gas is produced which turns limewater cloudy |
| B | add dilute nitric acid and aqueous barium nitrate | white precipitate forms |
| C | add dilute nitric acid and aqueous potassium manganate(VII) | solution decolourises |
| D | add dilute nitric acid and aqueous silver nitrate | white precipitate forms |
▶️ Answer/Explanation
Ans: B
The standard test for sulfate ions (SO42-) involves:
- First adding dilute nitric acid to remove any carbonate or sulfite ions that might interfere
- Then adding barium nitrate solution
- A positive result is the formation of a white precipitate of barium sulfate (BaSO4)
Why other options are incorrect:
- A: Gas turning limewater cloudy indicates carbonate, not sulfate
- C: Decolorization of manganate(VII) indicates a reducing agent, not specifically sulfate
- D: Silver nitrate tests for halides (chloride, bromide, iodide), forming white/slightly colored precipitates
The barium sulfate precipitate is particularly distinctive because it’s insoluble in both water and acids, making this a very reliable test for sulfate ions.
