Topic – 8.1
Fig. 1.1 shows part of the Periodic Table.
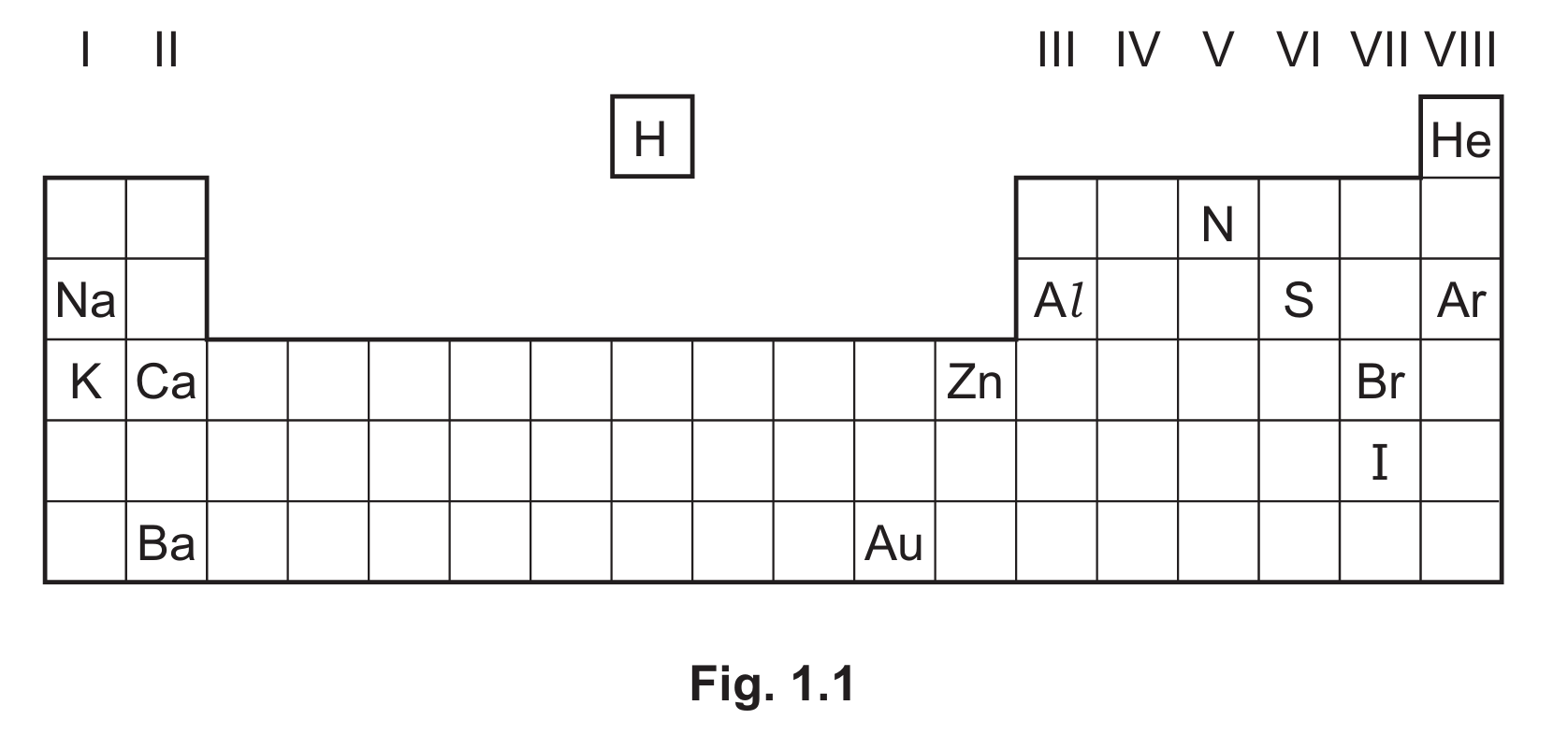
Answer the following questions using only the elements in Fig. 1.1. Each symbol of the element may be used once, more than once or not at all.
(a) Give the symbol of the element that:
(i) is 78% of clean, dry air
(ii) forms an ion with a charge of 3+
(iii) has an atom with only five occupied electron shells
(iv) forms an ion that gives a light green colour in a flame test
(v) is used in food containers because of its resistance to corrosion
(vi) is the metal with the lowest reactivity.
(b) Helium is a monatomic gas.
(i) State the meaning of the term monatomic.
(ii) Explain in terms of electronic configuration why helium is unreactive.
▶️ Answer/Explanation
(a)
(i) N (Nitrogen makes up about 78% of Earth’s atmosphere)
(ii) Al (Aluminum forms Al³⁺ ions by losing 3 electrons)
(iii) I (Iodine is in period 5, meaning its electrons occupy 5 shells)
(iv) Ba (Barium ions (Ba²⁺) produce a characteristic light green flame color)
(v) Al (Aluminum is used in food containers due to its corrosion-resistant oxide layer)
(vi) Au (Gold is the least reactive metal among the options)
(b)
(i) Monatomic means consisting of single atoms (not molecules). Helium exists as individual He atoms rather than as molecules like O₂ or N₂.
(ii) Helium is unreactive because it has a complete outer electron shell (2 electrons in its only shell, following the duplet rule). This stable configuration means it doesn’t need to gain, lose, or share electrons to become more stable.
Detailed Explanation:
For part (a), we need to carefully examine the periodic table fragment provided and recall key properties of the elements:
- Nitrogen (N) is the main component of air (78%)
- Aluminum (Al) is in group III and forms 3+ ions
- Iodine (I) is in period 5, meaning its electrons fill 5 shells (K, L, M, N, O)
- Barium (Ba) gives a light green flame test color
- Aluminum (Al) forms a protective oxide layer making it corrosion-resistant
- Gold (Au) is the least reactive metal shown (noble metal)
For part (b), helium’s monatomic nature and inertness are due to its complete valence shell (1s² configuration), which makes it extremely stable and unreactive under normal conditions.
Topic – 2.5
(a) Hydrogen chloride has a simple molecular structure.
(i) State two physical properties of a compound with a simple molecular structure.
(ii) Hydrogen chloride is a molecule with a covalent bond. Complete this sentence about a covalent bond. A covalent bond is formed when two atoms share a pair of …… .
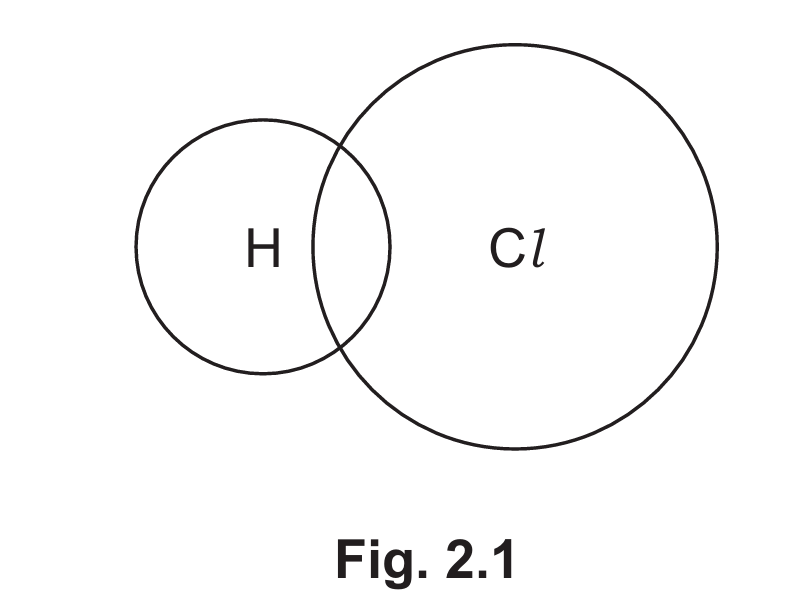
(iii) Complete Fig. 2.1 to show the dot-and-cross diagram for a molecule of hydrogen chloride. Show outer shell electrons only.
(b) Zinc chloride has a giant ionic structure of positive and negative ions. State the general name given to any negative ion.
(c) Diamond is used for jewellery.
(i) State one other use of diamond.
(ii) Choose the correct statement that describes the structure and bonding in diamond.
- simple covalent molecule
- giant covalent
- simple ionic
- giant ionic
▶️ Answer/Explanation
(a)(i) Two physical properties of simple molecular structures are:
- Low melting and boiling points (due to weak intermolecular forces)
- Poor electrical conductivity (as they don’t contain free electrons or ions)
(a)(ii) A covalent bond is formed when two atoms share a pair of electrons.
(a)(iii) The dot-and-cross diagram for HCl should show:
- One bonding pair of electrons between H and Cl (shared pair)
- Three lone pairs (6 electrons) on the chlorine atom
- No electrons on the hydrogen atom beyond the shared pair
(b) The general name for any negative ion is anion.
(c)(i) Another use of diamond is in cutting tools (due to its extreme hardness).
(c)(ii) The correct description of diamond’s structure is giant covalent (tick the second box). Diamond consists of carbon atoms each covalently bonded to four others in a giant three-dimensional lattice structure.
Topic – 10.1
(a) The list shows some substances present in water from natural sources.
dissolved oxygen
calcium compounds
plastics
harmful microbes
State which one of these substances provides essential minerals for aquatic life.
(b) Explain why phosphates present in polluted water are harmful to aquatic life.
(c) Table 3.1 shows the masses of ions, in mg, present in a 1000 cm³ sample of polluted water.

(i) Name the negative ion present in the highest concentration.
(ii) State the name of the NO₃⁻ ion.
(iii) Calculate the mass of phosphate ions present in 200 cm³ of polluted water.
(d) Fig. 3.1 shows some of the stages in the purification of drinking water.

(i) State the purpose of sedimentation.
(ii) State why chlorine is added to drinking water.
(e) Describe how to test for the purity of water using boiling point.
(f) Complete the symbol equation for the reaction of disulfur dichloride, \( S_2Cl_2 \), with water.
\[ S_2Cl_2 + ….H_2O \rightarrow ….HCl + H_2SO_3 + H_2S \]
▶️ Answer/Explanation
(a) calcium compounds
Calcium compounds provide essential minerals like calcium that are necessary for the growth and development of aquatic organisms, particularly for shell-forming creatures.
(b) (lead to) deoxygenation (of water)
Phosphates act as nutrients for algae, causing excessive algal growth (algal blooms). When these algae die, their decomposition by bacteria consumes large amounts of oxygen, leading to oxygen depletion in the water which can suffocate aquatic life.
(c)(i) hydrogencarbonate
With 10.0 mg in 1000 cm³, HCO₃⁻ has the highest concentration among negative ions in the table.
(c)(ii) nitrate
The NO₃⁻ ion is called nitrate, a common polyatomic ion in chemistry.
(c)(iii) 0.4 mg
Calculation: If 1000 cm³ contains 2.0 mg of phosphate ions, then 200 cm³ would contain (2.0 mg × 200)/1000 = 0.4 mg.
(d)(i) to remove solids
Sedimentation allows heavier solid particles to settle at the bottom of the water by gravity, making them easier to remove in subsequent filtration steps.
(d)(ii) to kill (harmful) microbes
Chlorine is added as a disinfectant to kill harmful bacteria, viruses and other pathogens that could cause waterborne diseases.
(e)
Method 1: Heat the water sample to its boiling point and measure the temperature. Pure water boils at exactly 100°C at standard pressure. If the boiling point is higher or occurs over a range of temperatures, the water is impure.
Method 2: Heat the sample to 100°C and observe if it boils. Pure water will boil exactly at 100°C, while impure water will either boil at a higher temperature or over a range of temperatures.
(f) \( 3H_2O \) and \( 2HCl \) (coefficients)
Balanced equation: \( S_2Cl_2 + 3H_2O \rightarrow 2HCl + H_2SO_3 + H_2S \)
Explanation: To balance the equation, we need 3 water molecules to provide enough oxygen and hydrogen atoms. This produces 2 HCl molecules while maintaining the balance of sulfur atoms (2 on each side).
Topic – 11.1
(a) Fig. 4.1 shows the displayed formula of compound A.
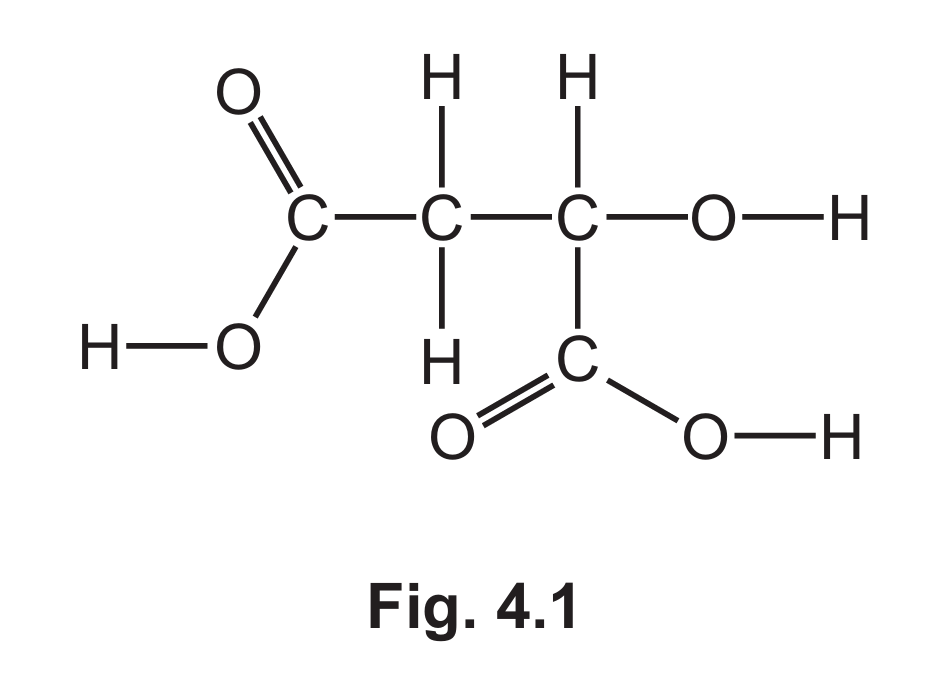
(i) On Fig 4.1 draw a circle around the alcohol functional group.
(ii) Deduce the molecular formula of compound A.
(b) Compound A reacts with ethanol to produce a compound with the molecular formula C8H14O5.
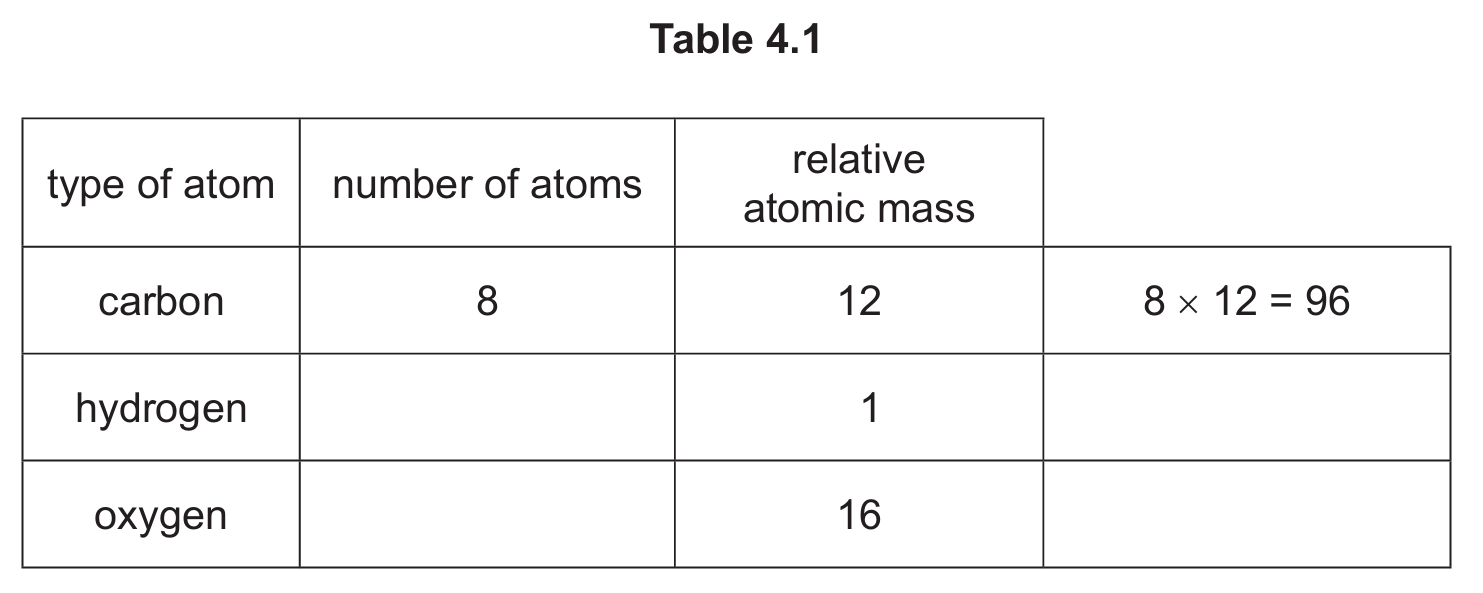
Complete Table 4.1 to calculate the relative molecular mass of C8H14O5.
(c) Complete the word equation for the complete combustion of ethanol.
ethanol + oxygen → …………………… + ……………………
(d) Table 4.2 shows the names, formulae and boiling points of ethene, propene, butene and pentene.
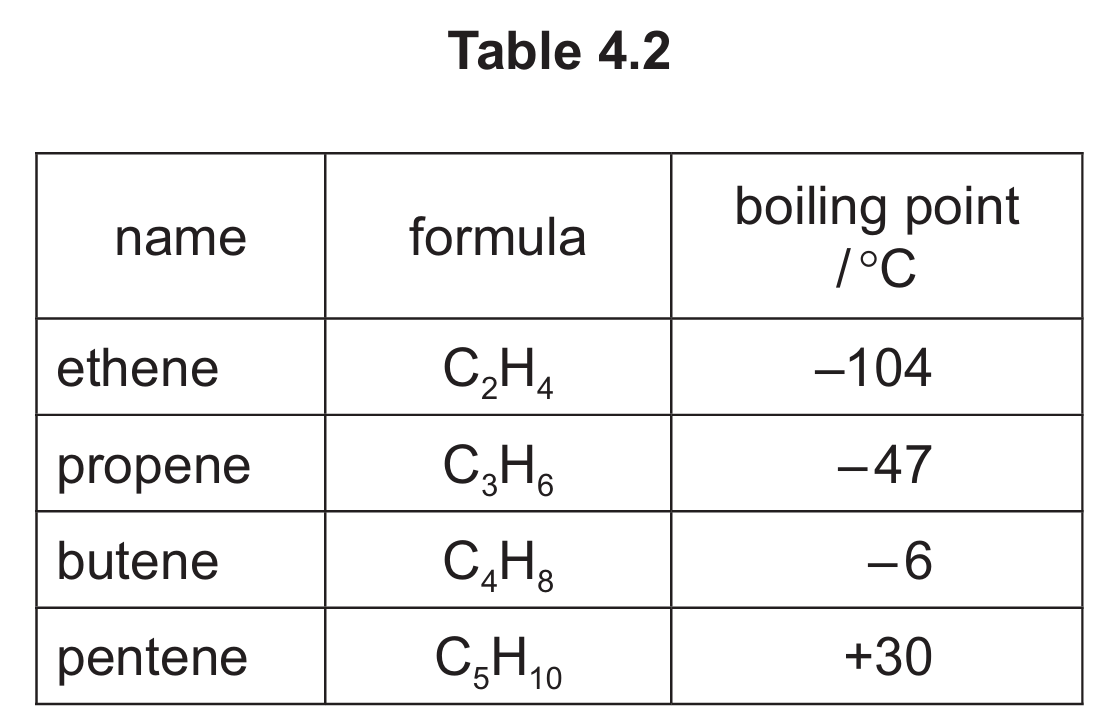
Use the information in Table 4.2 to answer these questions.
(i) Name the homologous series that includes ethene, propene, butene and pentene.
(ii) Deduce the general formula of this homologous series.
(iii) State the trend in the boiling point of this homologous series as the number of carbon atoms increases.
(e) Ethene is manufactured by cracking.
(i) Describe the manufacture of ethene by cracking.
(ii) Give a reason for cracking hydrocarbons.
▶️ Answer/Explanation
(a)(i) O-H group circled
The alcohol functional group is the hydroxyl group (-OH). In the displayed formula, this would be one of the O-H groups shown in the structure.
(a)(ii) C4H6O5
Counting all atoms in the displayed formula: 4 carbon atoms, 6 hydrogen atoms, and 5 oxygen atoms.
(b) 190
Calculation: (8 × 12) + (14 × 1) + (5 × 16) = 96 + 14 + 80 = 190
For hydrogen: 14 atoms × 1 = 14
For oxygen: 5 atoms × 16 = 80
(c) water + carbon dioxide
The complete combustion of ethanol follows the general pattern of hydrocarbon combustion: ethanol + oxygen → carbon dioxide + water.
(d)(i) alkenes
All these compounds contain a carbon-carbon double bond, which is the defining feature of the alkene homologous series.
(d)(ii) CnH2n
This is the general formula for alkenes, where n is the number of carbon atoms. Each compound in the series follows this pattern.
(d)(iii) increases
As the number of carbon atoms increases (from ethene to pentene), the boiling point increases (-104°C to +30°C). This is because larger molecules have stronger intermolecular forces.
(e)(i) Cracking involves breaking down larger hydrocarbon molecules into smaller ones (like ethene) using high temperatures and often a catalyst. The process typically uses long-chain alkanes from petroleum fractions.
Key points:
- Large alkane molecules are heated to high temperatures (400-900°C)
- Sometimes a catalyst (like aluminum oxide) is used
- Breaks C-C bonds to form smaller molecules including alkenes like ethene
- May also produce other useful products like hydrogen
(e)(ii) To produce more useful hydrocarbons that are in demand, particularly shorter chain alkenes like ethene which are important for making plastics and other chemicals.
Cracking converts less useful long-chain hydrocarbons into more valuable shorter-chain hydrocarbons that are in higher demand for industrial processes.
Topic – 8.3
(a) Table 5.1 shows some properties of five halogens.
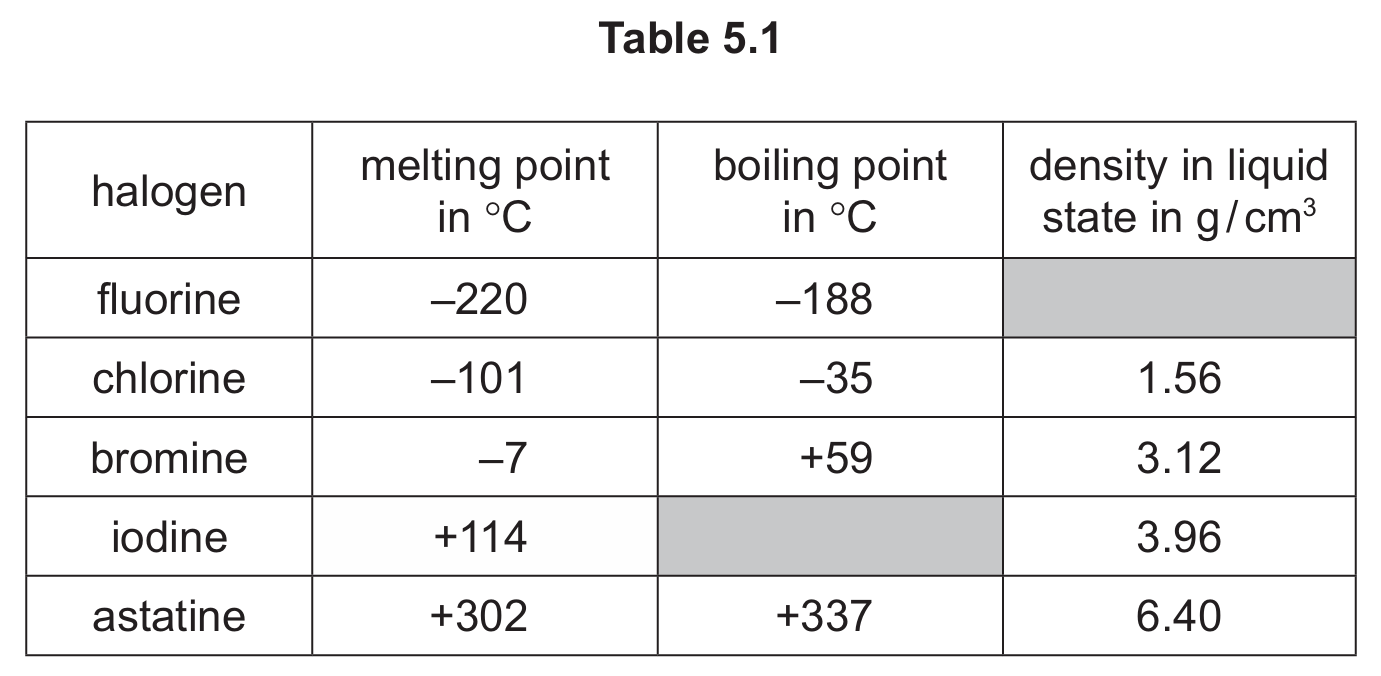
Use the information in Table 5.1 to predict:
(i) the boiling point of iodine
(ii) the density of liquid fluorine
(iii) the physical state of chlorine at -20°C. Give a reason for your answer.
(b) Aqueous chlorine reacts with aqueous lithium bromide.
(i) Complete the word equation for this reaction.
chlorine + lithium bromide → …………………… + ……………………
(ii) Explain why aqueous iodine does not react with aqueous lithium bromide.
(iii) Describe a test for chlorine.
(c) Fluorine reacts with ammonia to produce hydrogen fluoride and nitrogen.
Complete the symbol equation for this reaction.
…..F2 + 2NH3 → …..HF + N2
▶️ Answer/Explanation
(a)(i) Between 116°C and 335°C (inclusive)
Looking at the trend in boiling points down Group 17 (halogens), we see they increase with increasing atomic number. Iodine is between bromine (+59°C) and astatine (+337°C), so its boiling point should be in this range.
(a)(ii) Between 0.05 and 1.55 g/cm³ (inclusive)
The density increases down the group (chlorine: 1.56, bromine: 3.12, iodine: 3.96, astatine: 6.40). Fluorine, being the lightest halogen, should have the lowest density in this series.
(a)(iii) Gas (1 mark)
Boiling point is below -20°C / -20°C is above the boiling point (1 mark)
Chlorine’s boiling point is -35°C, so at -20°C (which is higher than -35°C), chlorine would be in its gaseous state.
(b)(i) bromine (1 mark)
lithium chloride (1 mark)
This is a displacement reaction where the more reactive chlorine displaces bromine from lithium bromide: Cl2 + 2LiBr → Br2 + 2LiCl
(b)(ii) Iodine is less reactive than bromine / bromine is more reactive than iodine / bromine is higher in the electrochemical series than iodine
In the reactivity series of halogens, chlorine > bromine > iodine. A halogen can only displace halide ions below it in the series, so iodine cannot displace bromine.
(b)(iii) damp litmus paper (1 mark)
is bleached (1 mark)
Chlorine is a powerful bleaching agent. When damp litmus paper is exposed to chlorine gas, it will first turn red (as chlorine is acidic) and then be bleached white.
(c) 3(F2) (1 mark)
6(HF) (1 mark)
The balanced equation is: 3F2 + 2NH3 → 6HF + N2
To balance: count atoms on both sides – we need 6 F atoms (so 3F2) and 6 H atoms (so 6HF).
Topic – 9.1
This question is about metals.
(a) Many metals have high melting points and boiling points.
State three other typical physical properties of metals.
(b) (i) Complete Table 6.1 to show the number of electrons, neutrons and protons in the sodium atom and silver ion shown.
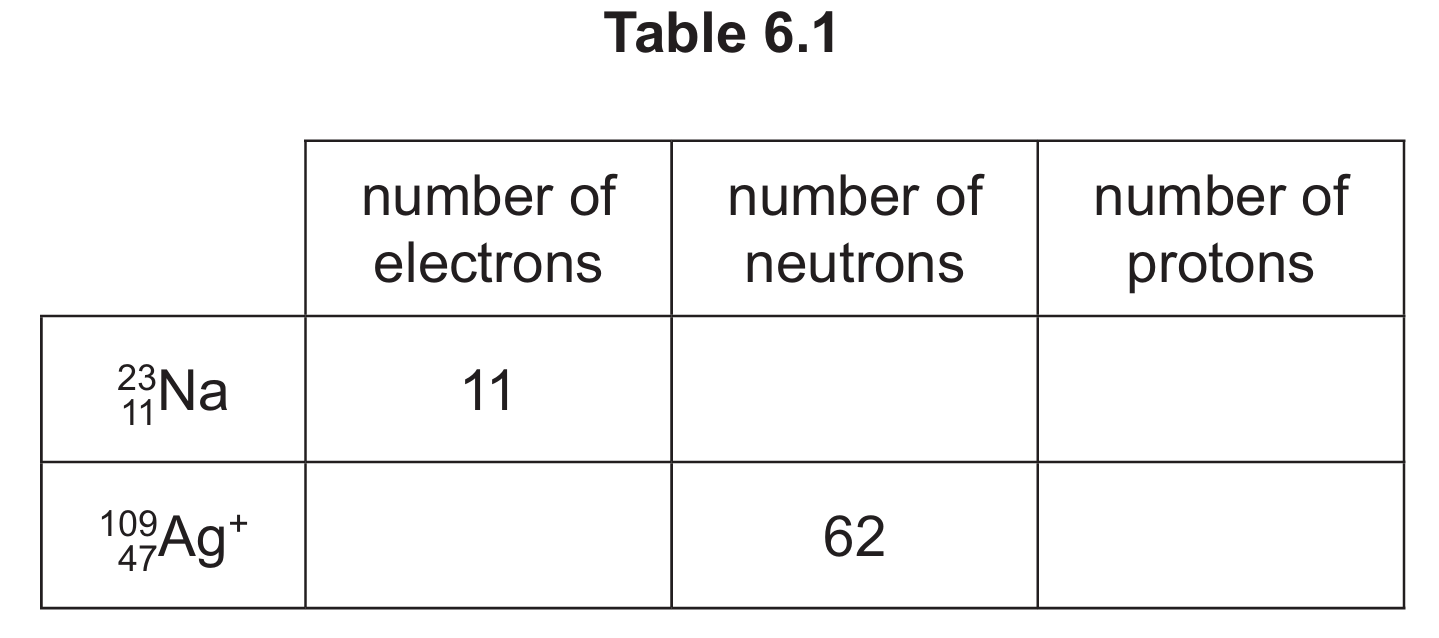
(ii) Write the electronic configuration of the sodium atom.
(c) Silver is a transition element. Sodium is in Group I of the Periodic Table.
State one difference in the physical properties of silver and sodium.
(d) Table 6.2 shows the observations when four different metals are heated in oxygen.
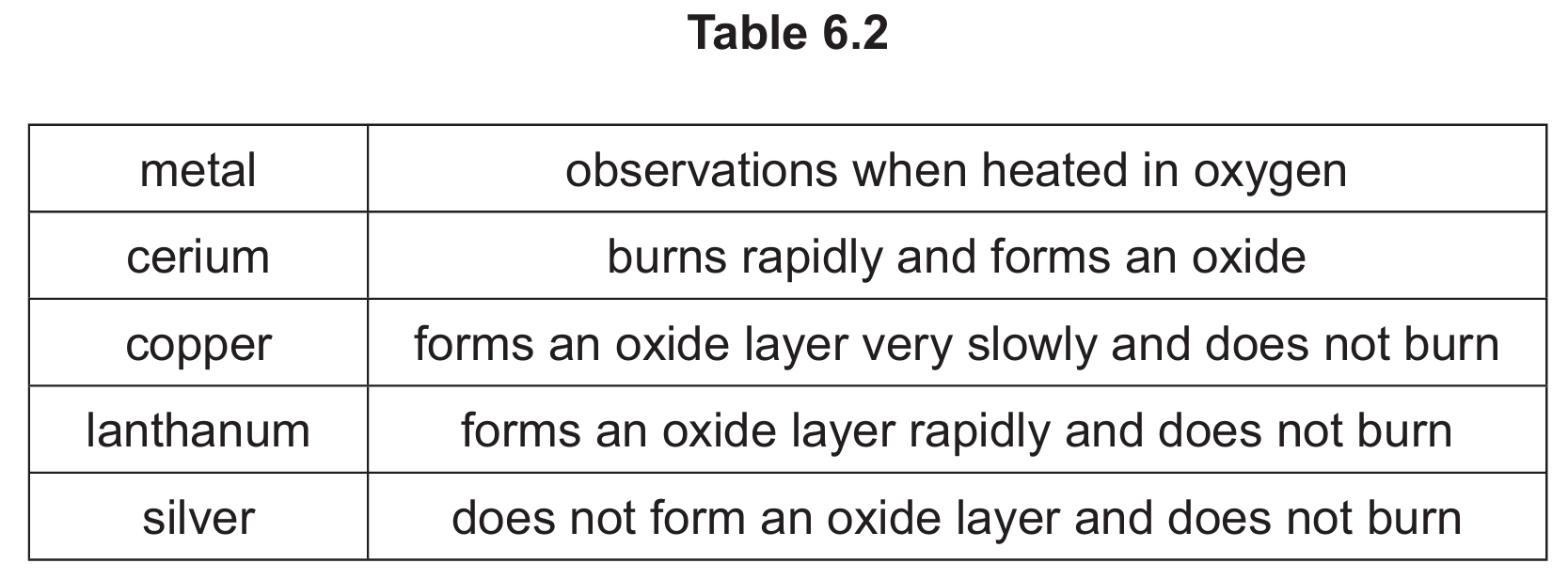
Put the four metals in order of their reactivity.
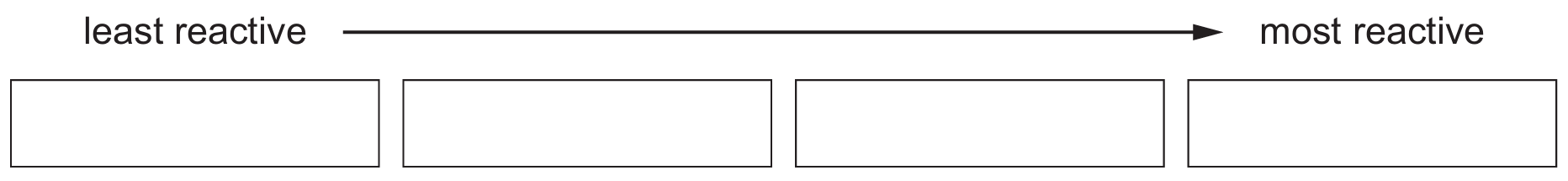
Put the least reactive metal first.
(e) Copper(II) oxide is reduced by carbon monoxide.
\( \text{CuO} + \text{CO} \rightarrow \text{Cu} + \text{CO}_2 \)
Explain how this equation shows that copper(II) oxide is reduced.
▶️ Answer/Explanation
(a) Any three of:
- Malleable (can be hammered into sheets)
- Ductile (can be drawn into wires)
- Good electrical conductors
- Good thermal conductors
- Shiny/lustrous appearance
- High density
(b)(i)
For sodium atom (\( \frac{23}{11}\text{Na} \)):
- Number of neutrons = Mass number – Atomic number = 23 – 11 = 12
- Number of protons = Atomic number = 11
For silver ion (\( \frac{109}{47}\text{Ag}^+ \)):
- Number of electrons = Atomic number – charge = 47 – 1 = 46
- Number of protons = Atomic number = 47
Completed table:
| number of electrons | number of neutrons | number of protons | |
|---|---|---|---|
| \( \frac{23}{11}\text{Na} \) | 11 | 12 | 11 |
| \( \frac{109}{47}\text{Ag}^+ \) | 46 | 62 | 47 |
(b)(ii) Electronic configuration of sodium atom: 2,8,1
(This represents the distribution of electrons in the K, L, and M shells respectively)
(c) Any one difference:
- Silver has higher density than sodium
- Silver has higher melting point than sodium
- Silver forms colored compounds while sodium compounds are typically white
- Silver is less reactive than sodium
(d) Order of reactivity: silver < copper < lanthanum < cerium
Explanation:
- Silver shows no reaction (least reactive)
- Copper reacts very slowly
- Lanthanum reacts rapidly but doesn’t burn
- Cerium burns rapidly (most reactive)
(e) Copper(II) oxide loses oxygen (to form copper metal), which is the definition of reduction.
In the reaction: CuO → Cu, the copper oxide is losing oxygen, so it’s being reduced. The carbon monoxide is acting as the reducing agent by removing oxygen from the copper oxide.
Topic – 7.3
(a) Crystals of zinc sulfate are made by warming excess solid zinc oxide with dilute sulfuric acid.
\[ \text{ZnO(s)} + \text{H}_2\text{SO}_4(\text{aq}) \rightarrow \text{ZnSO}_4(\text{aq}) + \text{H}_2\text{O(l)} \]
(i) State the meaning of the state symbol (aq).
(ii) State the method used to separate the excess solid zinc oxide from the reaction mixture.
(b) Crystals of sodium nitrate can be made by neutralising an acid with an alkali.
(i) Name the acid and the alkali used.
(ii) Complete the equation for all neutralisation reactions.
\[ \text{H}^+ + …… \rightarrow …… \]
(iii) Neutralisation reactions are exothermic.
Define the term exothermic.
(iv) Fig. 7.1 shows the reaction pathway diagram for an exothermic reaction.
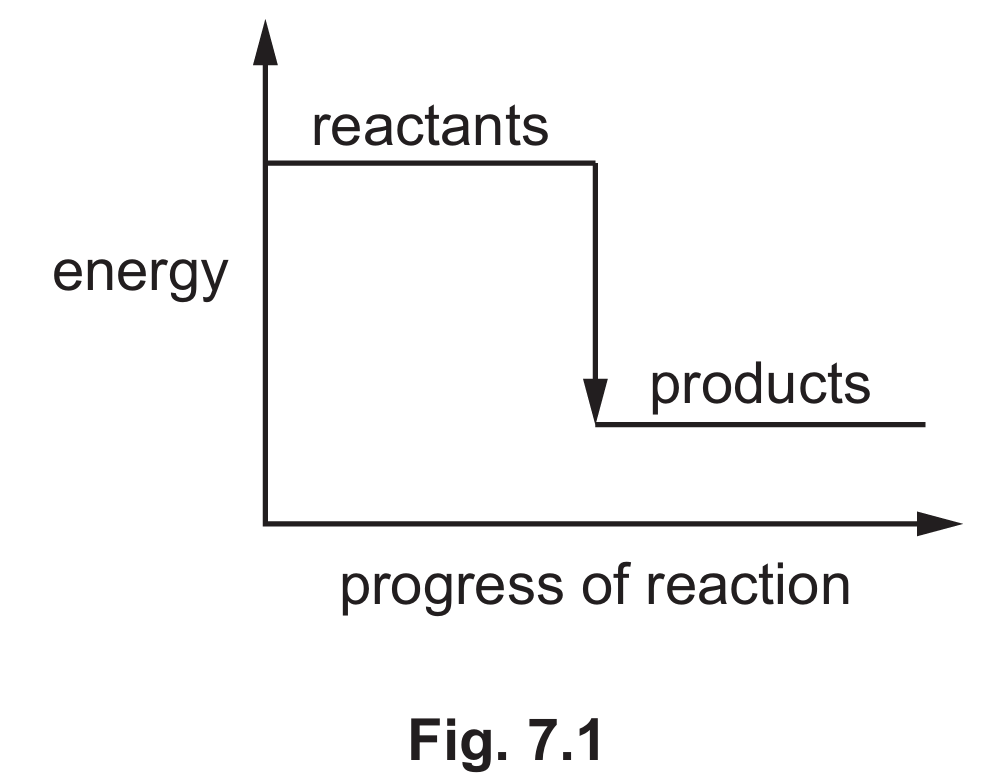
Explain how Fig. 7.1 shows that the reaction is exothermic.
(c) Methyl orange is an acid-base indicator.
State the colour of methyl orange at pH2 and at pH12.
▶️ Answer/Explanation
(a)(i) aqueous / dissolved in water
The state symbol (aq) indicates that the substance is dissolved in water, forming an aqueous solution.
(a)(ii) filtration
Filtration is used to separate the undissolved excess zinc oxide (solid) from the zinc sulfate solution.
(b)(i) acid: nitric acid; alkali: sodium hydroxide
Sodium nitrate is formed by the neutralization reaction between nitric acid (HNO₃) and sodium hydroxide (NaOH).
(b)(ii) \[ \text{H}^+ + \text{OH}^- \rightarrow \text{H}_2\text{O} \]
This is the ionic equation for all neutralization reactions, showing the combination of hydrogen ions and hydroxide ions to form water.
(b)(iii) release of thermal energy
An exothermic reaction is one that releases energy, usually in the form of heat, to the surroundings.
(b)(iv) The energy of the products is lower than the energy of the reactants.
In an exothermic reaction, the products have less energy than the reactants, with the difference being released as heat. The diagram shows this by having the products at a lower energy level than the reactants.
(c) pH2: red/pink; pH12: yellow
Methyl orange is a pH indicator that changes color depending on the acidity. In strongly acidic conditions (pH2), it appears red/pink, while in strongly alkaline conditions (pH12), it turns yellow. The transition range is between pH 3.1 (red) and pH 4.4 (yellow).
Topic – 6.2
(a) A student investigates the reaction of small pieces of calcium carbonate with excess dilute hydrochloric acid of three different concentrations. The time taken for each reaction to finish is recorded.
The three concentrations of acid are:
- 0.5 mol/dm3
- 1.0 mol/dm3
- 2.0 mol/dm3
All other conditions stay the same.
Table 8.1 shows the time taken for each reaction to finish.

(i) Complete Table 8.1 by writing the concentrations in the first column.
(ii) Describe the effect on the time taken for the reaction to finish when the reaction is carried out at a lower temperature. All other conditions stay the same.
(iii) Describe the effect on the time taken for the reaction to finish when powdered calcium carbonate is used instead of small pieces of calcium carbonate. All other conditions stay the same.
(b) Molten calcium chloride is electrolysed using inert electrodes.
(i) Name the products at the positive and negative electrodes.
(ii) Choose from the list the substance that is used as an inert electrode. Draw a circle around your chosen answer.
graphite iodine magnesium phosphorus
(c) Carbon dioxide is a gas at room temperature. Describe the motion and separation of the particles in carbon dioxide gas.
▶️ Answer/Explanation
(a)(i) The completed table should be:
- 1.0 mol/dm3 – 32 s
- 0.5 mol/dm3 – 64 s
- 2.0 mol/dm3 – 16 s
This shows that higher concentrations lead to faster reactions (shorter times).
(a)(ii) At a lower temperature, the time taken for the reaction to finish would increase (longer time). This is because lower temperatures decrease the kinetic energy of particles, resulting in fewer successful collisions per second between reactant particles.
(a)(iii) Using powdered calcium carbonate instead of small pieces would decrease the time taken for the reaction to finish (shorter time). This is because powdering increases the surface area of the solid reactant, providing more contact points for collisions with acid particles.
(b)(i) The products are:
- Positive electrode (anode): Chlorine (Cl2)
- Negative electrode (cathode): Calcium (Ca)
This is because during electrolysis of molten calcium chloride, calcium ions (Ca2+) are reduced at the cathode to form calcium metal, while chloride ions (Cl–) are oxidized at the anode to form chlorine gas.
(b)(ii) The correct inert electrode is graphite. Graphite is commonly used as an inert electrode because it conducts electricity well and doesn’t react with the products of electrolysis.
(c) In carbon dioxide gas at room temperature:
- Motion: The particles move rapidly in random, irregular directions with no fixed pattern.
- Separation: The particles are far apart from each other relative to their size, with large spaces between them.
This explains why gases are compressible and can expand to fill their containers.
