Topic – 8.2
Fig. 1.1 shows part of the Periodic Table.

(a) Answer the following questions using only the elements in Fig. 1.1. Each symbol of the element may be used once, more than once or not at all. Give the symbol of the element that:
(i) is in brass
(ii) produces an orange-red colour in a flame test
(iii) is a reactant in a fuel cell
(iv) has an atom with only three occupied electron shells
(v) forms an ion that gives a red-brown precipitate on addition of aqueous ammonia
(vi) forms an ion with a charge of 1-.
(b) Explain why Li, K and Rb have similar chemical properties.
▶️ Answer/Explanation
(a)
(i) Zn – Brass is an alloy of copper and zinc.
(ii) Ca – Calcium produces an orange-red flame color in flame tests.
(iii) H – Hydrogen is a reactant in fuel cells, combining with oxygen to produce electricity.
(iv) Al – Aluminum has electrons in three shells (2,8,3).
(v) Fe – Iron(III) ions form a red-brown precipitate with aqueous ammonia.
(vi) I – Iodine forms I⁻ ions with a 1- charge.
(b) Li, K and Rb have similar chemical properties because they are all in Group I (alkali metals) of the periodic table. This means they have the same number of electrons in their outer shell (1 electron each). Elements in the same group have similar chemical properties because chemical reactions primarily involve the outer shell electrons, and when these are the same, the elements behave similarly in chemical reactions.
Detailed Explanation:
1. For part (a), we need to identify elements based on their specific properties:
- Brass contains zinc (Zn) along with copper
- Calcium (Ca) gives characteristic orange-red color in flame tests
- Hydrogen (H) is used in fuel cells
- Aluminum (Al) has three electron shells occupied (n=1,2,3)
- Iron (Fe) forms Fe³⁺ which gives red-brown precipitate with NH₃
- Iodine (I) forms I⁻ ions commonly
2. For part (b), the key points are:
- Group I elements have identical outer electron configuration (ns¹)
- This similar electron configuration leads to similar chemical behavior
- All readily lose one electron to form +1 ions
- All react vigorously with water to form alkaline solutions
The periodic trend explains why elements in the same group behave similarly despite having different numbers of electron shells.
Topic – 2.5
Oxygen, water and ethene have simple molecular structures.
(a) (i) State the percentage of oxygen in clean, dry air.
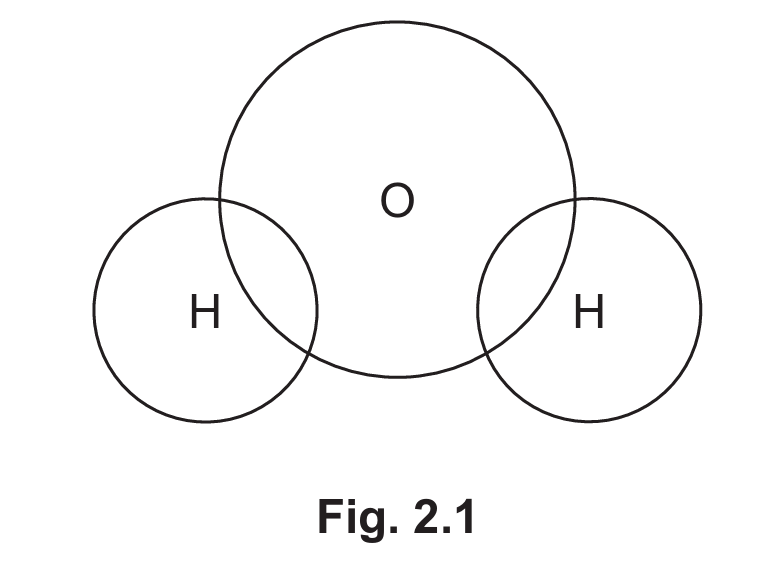
(ii) Complete Fig. 2.1 to show the dot-and-cross diagram for a molecule of water. Show outer shell electrons only.
(iii) Ethene is a small molecule used to make polymers. State the name of the polymer formed from ethene.
(iv) Complete this sentence about polymers. Polymers are large molecules built up from many smaller molecules called ________.
(b) Potassium chloride is an ionic compound.
(i) State two physical properties of ionic compounds.
(ii) Choose the correct statement that describes ionic bonding.
□ It is a weak electrostatic attraction between anions and cations.
□ It is a weak electrostatic attraction between cations.
□ It is a strong electrostatic attraction between anions.
□ It is a strong electrostatic attraction between cations and anions.
▶️ Answer/Explanation
(a) (i) 21%
The atmosphere contains approximately 21% oxygen by volume in clean, dry air.
(ii) The correct dot-and-cross diagram for water should show:
– Two hydrogen atoms each sharing one electron with oxygen (single covalent bonds)
– Oxygen with two lone pairs (four non-bonding electrons)
– No extra electrons on hydrogen atoms
(iii) poly(ethene)
Ethene monomers (C₂H₄) polymerize to form the addition polymer poly(ethene), commonly known as polyethylene.
(iv) monomers
Polymers are formed by linking together many smaller monomer units through chemical reactions.
(b) (i) Any two of:
– High melting points
– Conduct electricity when molten or in aqueous solution
– Soluble in water
– Form crystalline structures
Ionic compounds have strong electrostatic forces between ions, leading to high melting points. Their ions become mobile when molten or dissolved, allowing conductivity.
(ii) The correct statement is: “It is a strong electrostatic attraction between cations and anions.”
Ionic bonding involves the strong electrostatic attraction between positively charged cations and negatively charged anions, formed by electron transfer.
Topic – 10.1
(a) The list shows some gases in a sample of water. Choose from the list the gas that is essential for aquatic life. Draw a circle around your chosen answer.
argon hydrogen nitrogen oxygen
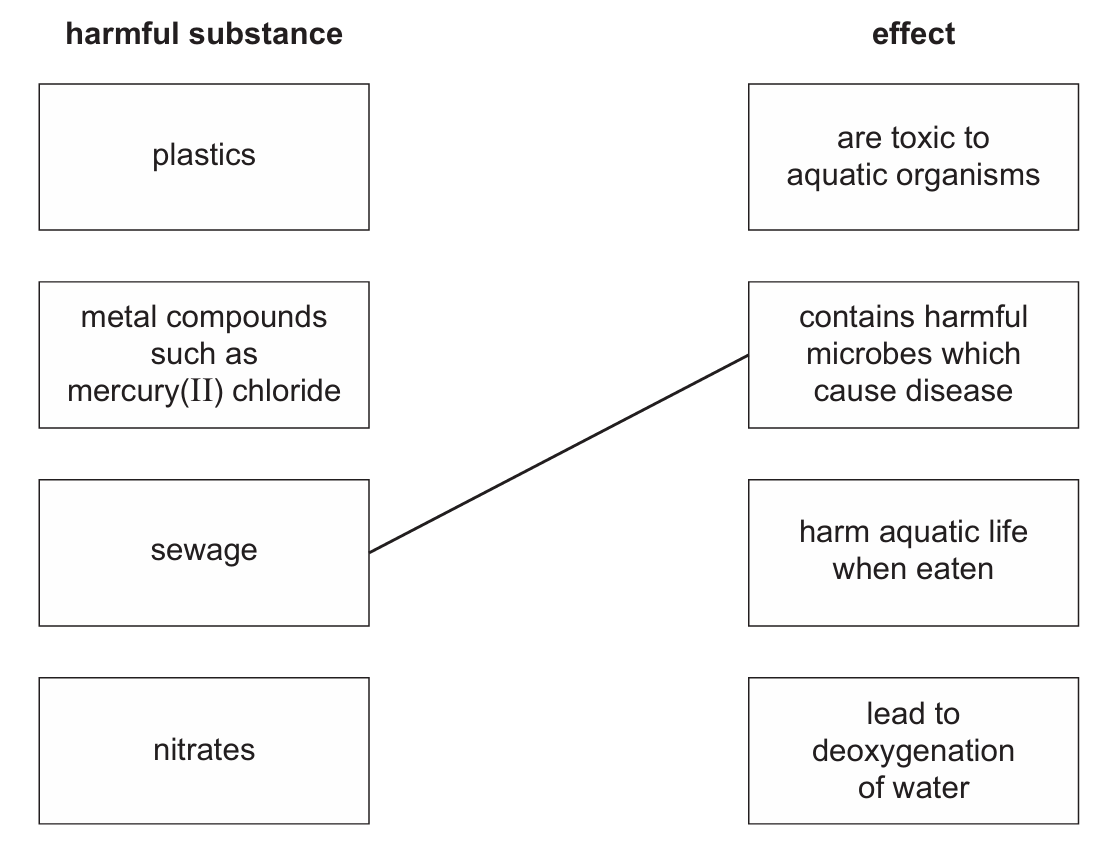
(b) Polluted water contains harmful substances. Link each harmful substance on the left to the correct effect on the right. One has been done for you.
(c) Table 3.1 shows the masses of ions, in mg, present in a 1000 cm3 sample of polluted water.
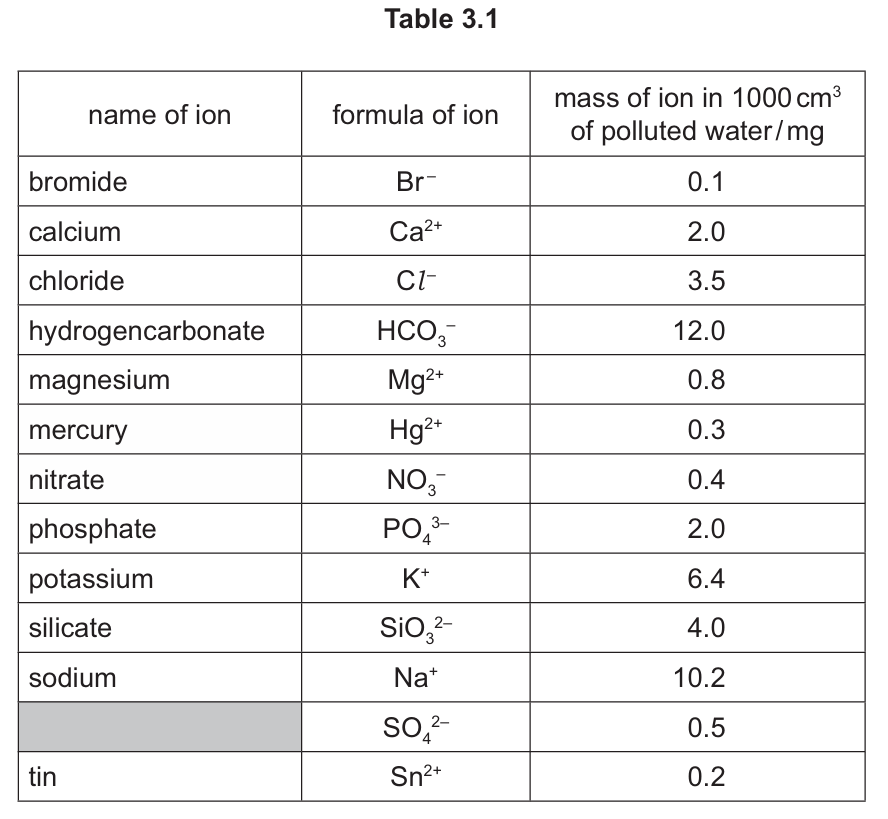
(i) Name the positive ion that has the lowest concentration.
(ii) State the name of the SO42- ion.
(iii) Calculate the mass of potassium ions in 125 cm3 of polluted water.
(d) Name two substances used in the treatment of the domestic water supply. For each substance give a reason for its use.
(e) Complete the symbol equation for the reaction of silicon(IV) chloride, SiCl4, with water.
SiCl4 + ….H2O → SiO2 + ….HCl
▶️ Answer/Explanation
(a) oxygen
Oxygen is essential for aquatic life as it is required for respiration by fish and other aquatic organisms.
(b) Correct pairings:
– metal compounds → are toxic to aquatic organisms
– plastics → harm aquatic life when eaten
– nitrates → lead to deoxygenation of water
– sewage → contains harmful microbes which cause disease
(c)(i) tin
Sn2+ has the lowest concentration at 0.2 mg per 1000 cm3 among the positive ions listed.
(c)(ii) sulfate
The SO42- ion is called sulfate, a common polyatomic ion.
(c)(iii) 0.8 mg
Calculation: (6.4 mg/1000 cm3) × 125 cm3 = 0.8 mg. This is a simple proportion calculation.
(d) Possible answers:
– Carbon: to remove tastes and odors
– Chlorine: to kill harmful microbes
– Fluoride: to help prevent tooth decay
– Alum: to coagulate suspended particles
(e) SiCl4 + 2H2O → SiO2 + 4HCl
Balancing the equation: We need 2 water molecules to provide enough oxygen atoms for SiO2, which produces 4 HCl molecules to balance the chlorine atoms.
Topic – 11.6
(a) Fig. 4.1 shows the displayed formula of compound A.
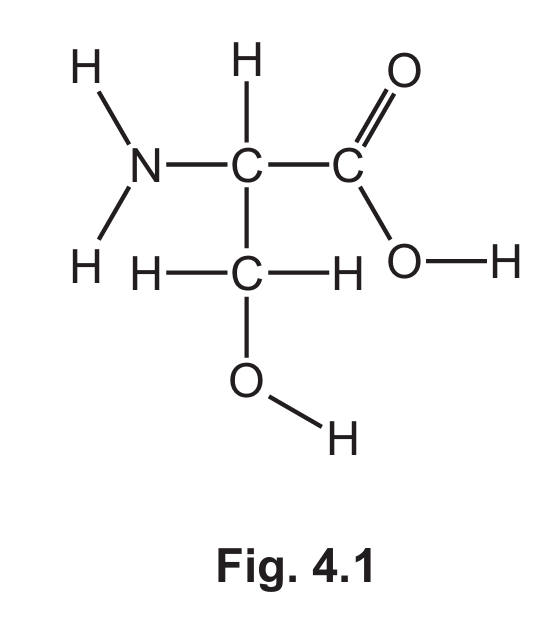
(i) On Fig 4.1 draw a circle around the carboxylic acid functional group.
(ii) Deduce the molecular formula of compound A.
(b) Compound A reacts with ethanol to produce a compound with the molecular formula \( C_5H_{11}NO_3 \).
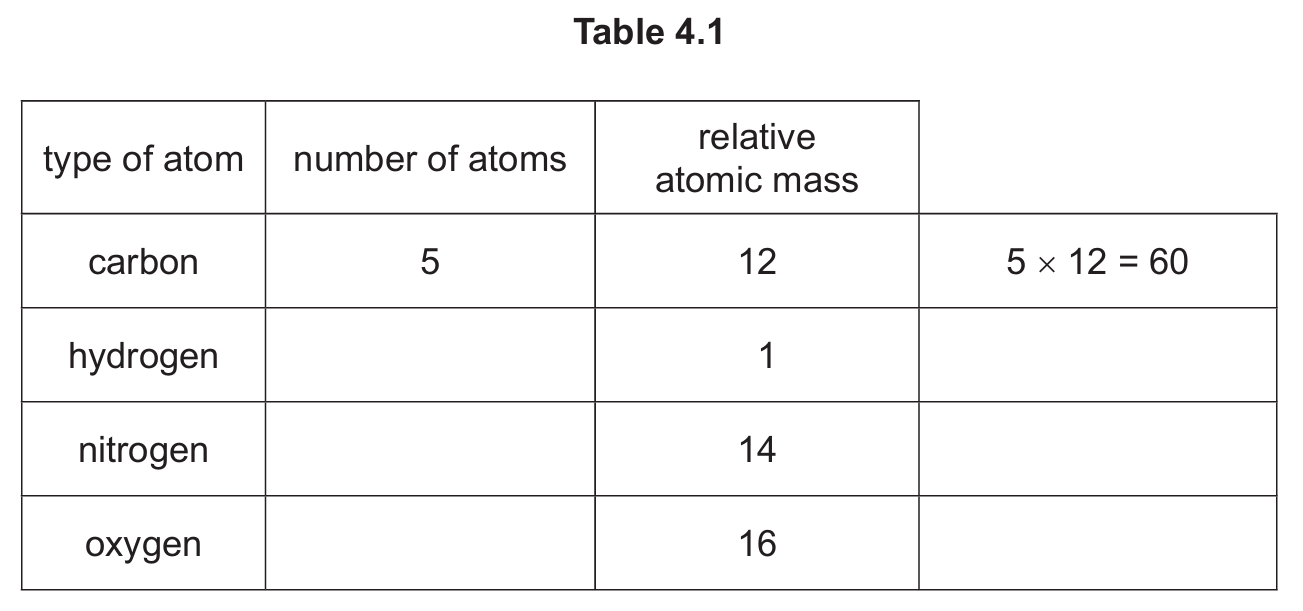
Complete Table 4.1 to calculate the relative molecular mass of \( C_5H_{11}NO_3 \).
(c) Table 4.2 shows the names, formulae and boiling points of methanol, ethanol, propanol and butanol.
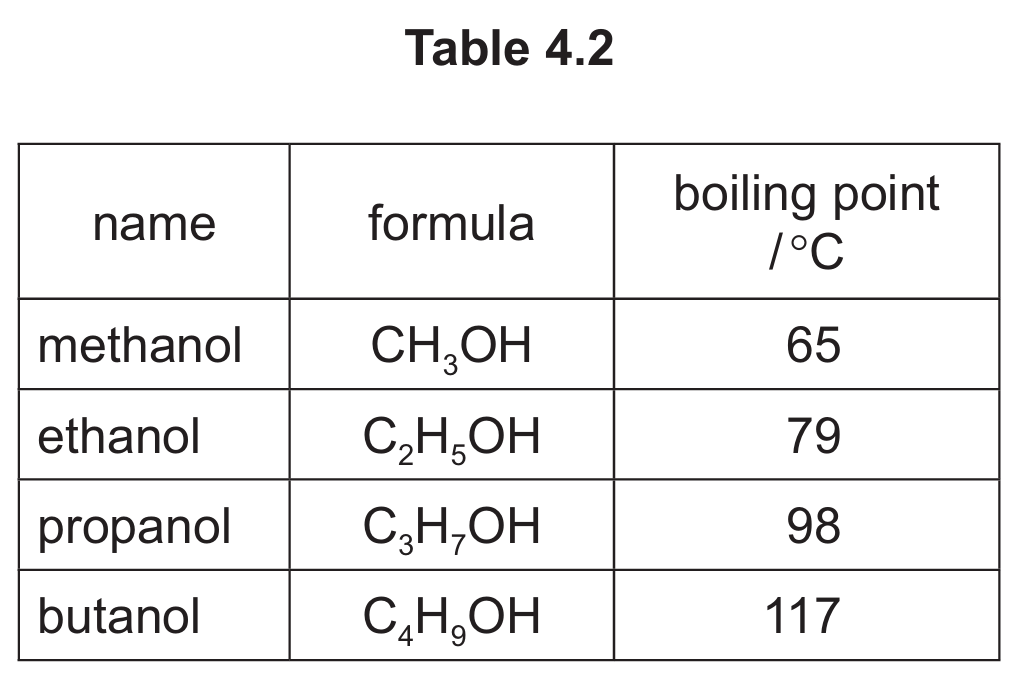
Use the information in Table 4.2 to answer these questions.
(i) Name the homologous series that includes methanol, ethanol, propanol and butanol.
(ii) Deduce the general formula of this homologous series.
(iii) State the trend in the boiling point of this homologous series as the number of carbon atoms increases.
(d) Ethanol can be manufactured by an addition reaction.
(i) Name two substances and state two conditions required.
(ii) Draw the displayed formula of ethanol.
(iii) Name the toxic gas produced when ethanol undergoes incomplete combustion.
▶️ Answer/Explanation
(a)(i) COOH group circled
The carboxylic acid functional group is -COOH. In the displayed formula, this group should be clearly identified and circled.
(a)(ii) \( C_3H_7NO_3 \)
By counting all atoms in the displayed formula of compound A, we find it contains 3 carbon atoms, 7 hydrogen atoms, 1 nitrogen atom, and 3 oxygen atoms.
(b) 133
Calculation:
Carbon: 5 × 12 = 60
Hydrogen: 11 × 1 = 11
Nitrogen: 1 × 14 = 14
Oxygen: 3 × 16 = 48
Total = 60 + 11 + 14 + 48 = 133
(c)(i) alcohol(s)
All these compounds contain the -OH functional group, which defines the alcohol homologous series.
(c)(ii) \( C_nH_{2n+1}OH \)
This is the general formula for alcohols, where n represents the number of carbon atoms.
(c)(iii) Increases
As the number of carbon atoms increases, the boiling point increases due to stronger van der Waals forces between larger molecules.
(d)(i)
Substances required:
• ethene
• steam/water
Conditions:
• 300°C temperature
• 6000 kPa/60 atm pressure
• phosphoric acid catalyst
(d)(ii) 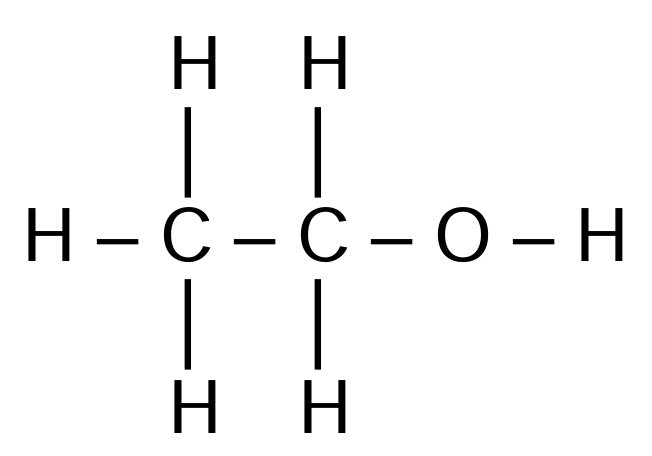
(d)(iii) carbon monoxide
Incomplete combustion of ethanol occurs when there’s insufficient oxygen, producing toxic carbon monoxide gas instead of carbon dioxide.
Topic – 8.3
(a) Table 5.1 shows some properties of five halogens.
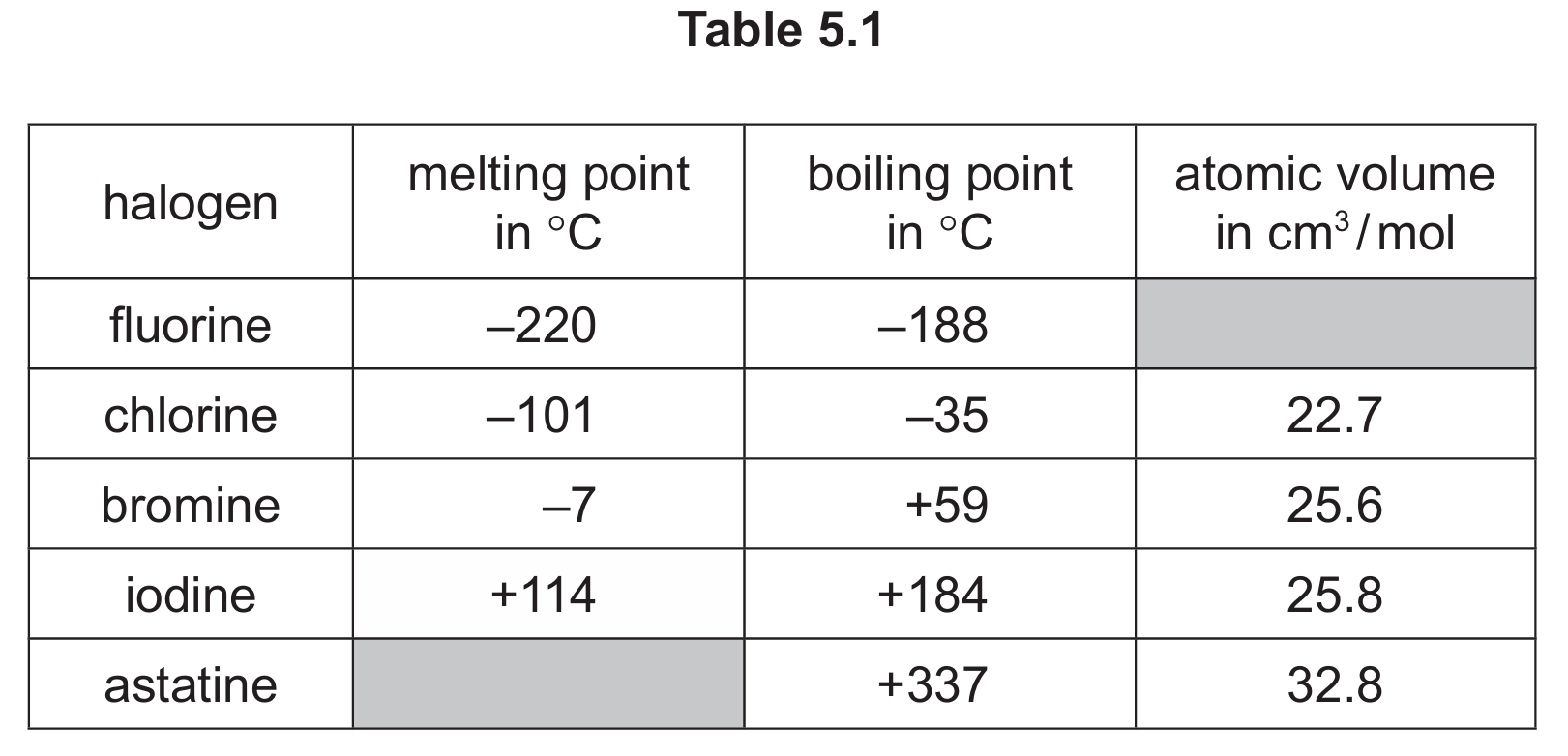
Use the information in Table 5.1 to predict:
(i) the melting point of astatine
(ii) the atomic volume of fluorine
(iii) the physical state of fluorine at −240°C. Give a reason for your answer.
(b) Aqueous chlorine reacts with aqueous sodium iodide.
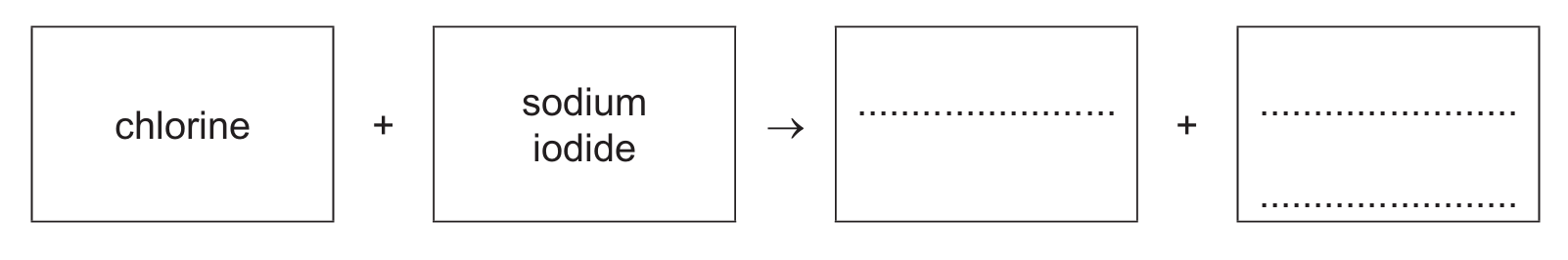
(i) Complete the word equation for this reaction.
(ii) Explain why aqueous bromine does not react with aqueous sodium chloride.
(c) Fluorine reacts with water to produce hydrogen fluoride and oxygen.
Complete the symbol equation for this reaction.
\[ 2F_2 + \ldots H_2O \rightarrow 4HF + \ldots \]
(d) Name an anhydrous compound used to test for water. State the colour of the compound after water is added.
▶️ Answer/Explanation
(a)(i) Between 116°C and 335°C (inclusive)
Looking at the trend in melting points down Group 17 (halogens), we see they increase: F (-220°C), Cl (-101°C), Br (-7°C), I (114°C). Astatine, being below iodine, would have a higher melting point than iodine but lower than its boiling point of 337°C.
(a)(ii) Less than 22.7 cm³/mol (but not below 1.0)
The atomic volume increases down the group: Cl (22.7), Br (25.6), I (25.8), At (32.8). Fluorine, being above chlorine, would have a smaller atomic volume.
(a)(iii) Solid (1), because −240°C is below fluorine’s melting point of −220°C (1)
At temperatures below its melting point, a substance exists as a solid. Since −240°C is 20 degrees below fluorine’s melting point, it would be solid.
(b)(i) iodine (1) + sodium chloride (1)
This is a displacement reaction where the more reactive chlorine displaces iodine from sodium iodide: Cl₂ + 2NaI → I₂ + 2NaCl
(b)(ii) Bromine is less reactive than chlorine / chlorine is more reactive than bromine (1)
In the reactivity series of halogens, chlorine is above bromine, meaning it can displace bromine but bromine cannot displace chlorine. Therefore, bromine cannot displace chlorine from sodium chloride.
(c) \[ 2F_2 + 2H_2O \rightarrow 4HF + O_2 \] (2 marks: 1 for each correct coefficient)
The reaction shows fluorine reacting with water to produce hydrogen fluoride and oxygen. Balancing the equation requires 2 water molecules to produce 1 oxygen molecule while maintaining the hydrogen and fluorine counts.
(d) Copper(II) sulfate (1) → blue (1) OR Cobalt(II) chloride (1) → red/pink (1)
Anhydrous copper(II) sulfate is white and turns blue when hydrated. Anhydrous cobalt(II) chloride is blue and turns pink when hydrated. Both are common tests for water.
Topic – 9.1
This question is about metals.
(a) Metals are malleable and ductile.
State three other typical physical properties of metals.
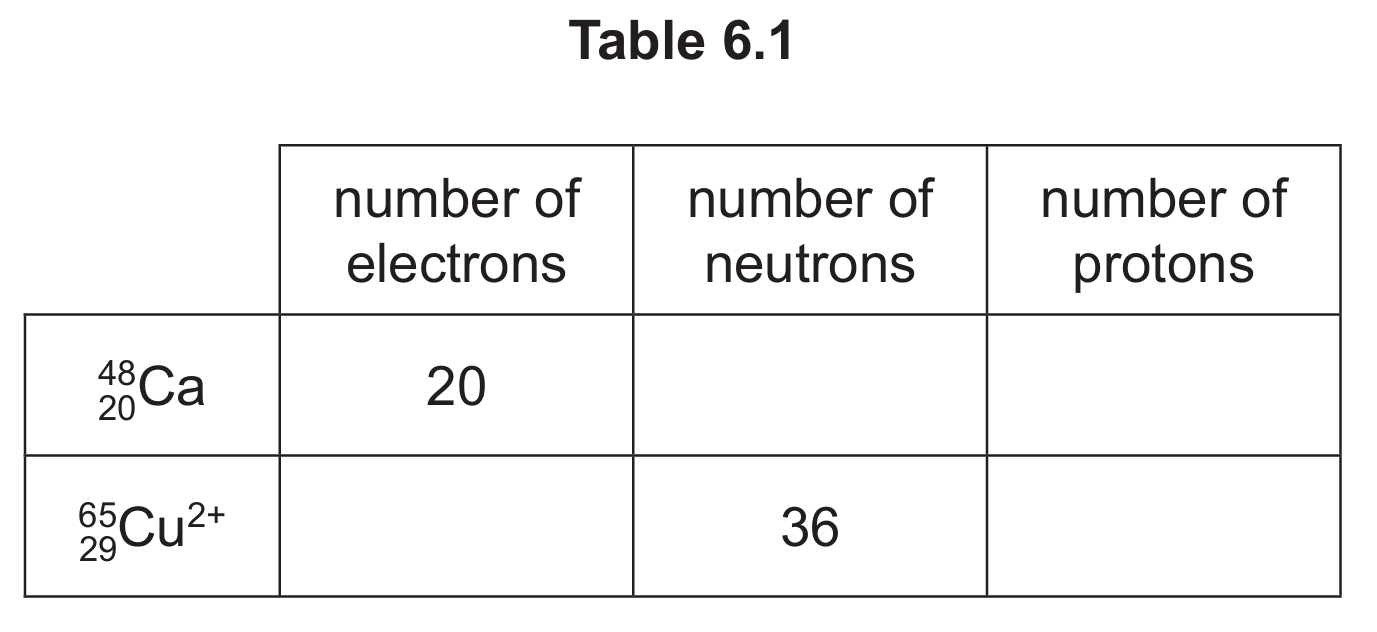
(b) (i) Complete Table 6.1 to show the number of electrons, neutrons and protons in the calcium atom and copper ion shown.
(ii) Write the electronic configuration of the calcium atom.
(c) Copper is a transition element.
Choose the correct statement about transition elements.
They have low densities.
They often act as catalysts.
They have low melting points.
All their compounds are white.
(d) Table 6.2 shows the observations when four different metals react with concentrated nitric acid.
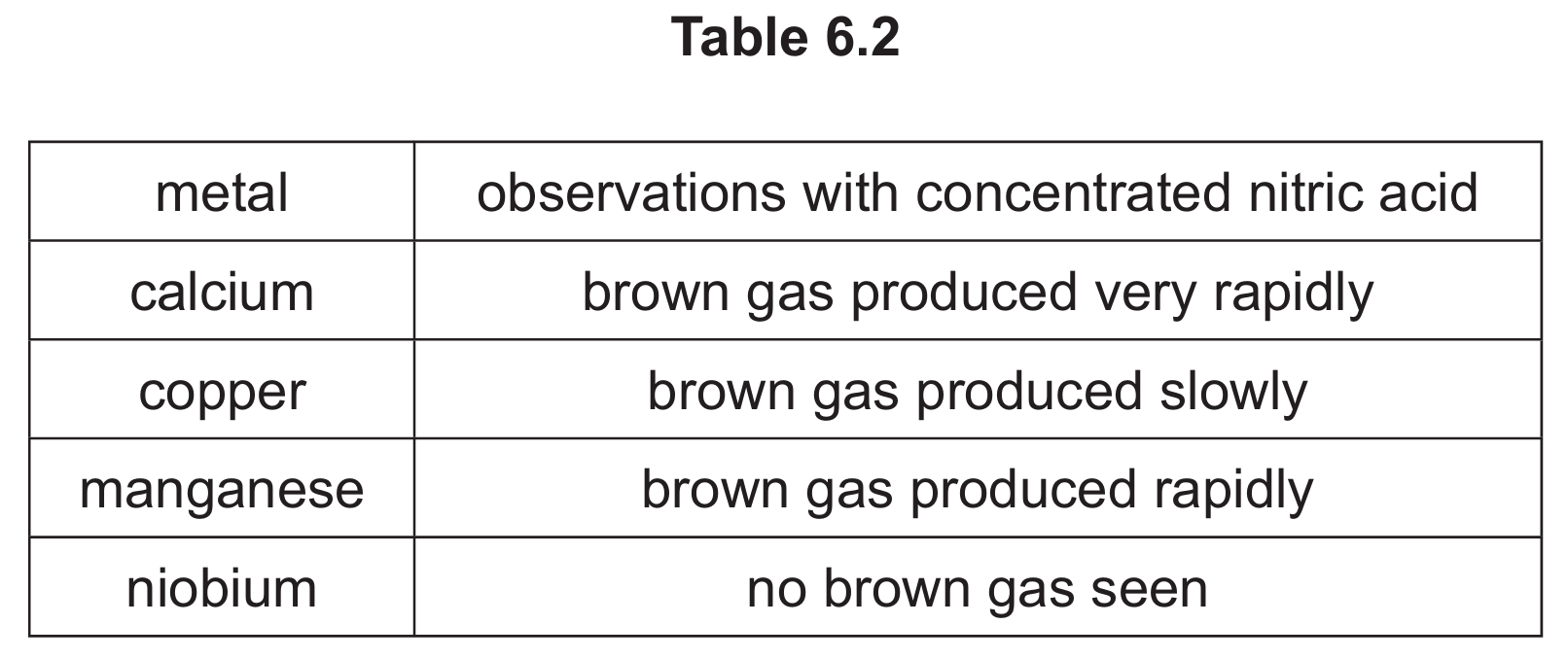
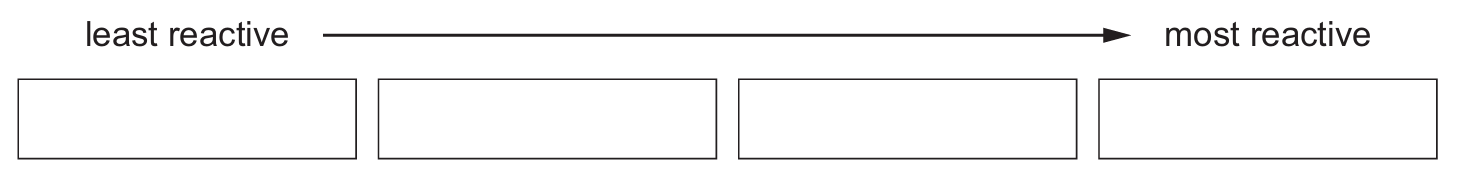
Put the four metals in order of their reactivity.
Put the least reactive metal first.
(e) Manganese(IV) oxide is reduced by aluminium.
\[3MnO_2 + 4Al \rightarrow 3Mn + 2Al_2O_3\]
Explain how this equation shows that manganese(IV) oxide is reduced.
▶️ Answer/Explanation
(a) 1. high melting point(s) / high boiling point(s)
2. electrical conductor
3. thermal conductor
Metals typically have high melting and boiling points due to strong metallic bonds. They are good conductors of electricity because of delocalized electrons that can move freely. Their thermal conductivity comes from both these mobile electrons and the closely packed lattice structure.
(b)(i) number of neutrons in Ca = 28
number of electrons in Cu2+ = 27
number of protons in Ca = 20 AND number of protons in Cu2+ = 29
For 48Ca: neutrons = mass number – atomic number = 48 – 20 = 28. For 65Cu2+: protons = atomic number = 29, electrons = 29 – 2 = 27 (as it’s a 2+ ion).
(b)(ii) 2,8,8,2
Calcium (atomic number 20) has electron configuration: 2 electrons in first shell, 8 in second, 8 in third, and 2 in fourth shell.
(c) They often act as catalysts.
Transition elements are known for their catalytic properties (e.g., iron in Haber process, nickel in hydrogenation). While they do have high densities and melting points, and form colored compounds, these aren’t universal properties.
(d) niobium < copper < manganese < calcium
The reactivity order is determined by the rate of brown gas (NO2) production: no gas (least reactive) → slow production → rapid production → very rapid production (most reactive).
(e) manganese(IV) oxide loses oxygen
Reduction is gain of electrons or loss of oxygen. In this reaction, MnO2 loses oxygen to form Mn, while aluminium gains oxygen to form Al2O3 (oxidation).
Topic – 7.1
This question is about acids, bases and salts.
(a) Crystals of zinc chloride can be made by warming excess solid zinc oxide with dilute hydrochloric acid.
\[ \text{ZnO(s)} + 2\text{HCl(aq)} \rightarrow \text{ZnCl}_2\text{(aq)} + \text{H}_2\text{O(…….)} \]
(i) Complete the symbol equation by adding the state symbol for water at room temperature.
(ii) State the method used to separate the excess solid zinc oxide from the reaction mixture.
(iii) Describe how to make dry crystals of zinc chloride from an aqueous solution of zinc chloride.
(b) Choose from the list the ion that is present in all acids.
Draw a circle around your chosen answer.
Cl– H+ O2- OH–
(c) The reaction of zinc oxide with hydrochloric acid is exothermic.
(i) Define the term exothermic.
(ii) Fig. 7.1 shows the incomplete reaction pathway diagram for the reaction of zinc oxide with hydrochloric acid.
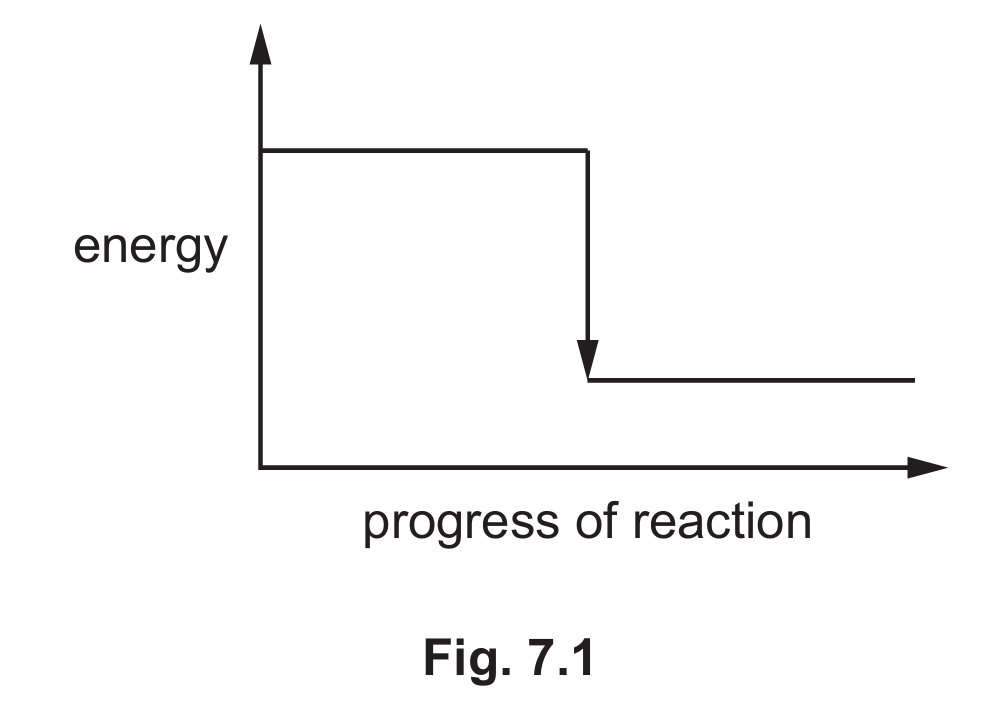
Complete Fig. 7.1 by writing these formulae on the diagram:
• ZnO + 2HCl
• ZnCl2 + H2O.
(iii) Explain how Fig. 7.1 shows that the reaction is exothermic.
(d) Litmus is an acid-base indicator.
State the colour of litmus at pH 2 and at pH 12.
▶️ Answer/Explanation
(a)(i) (l)
Water at room temperature is in liquid state, so the state symbol is (l).
(a)(ii) filtration
Filtration is used to separate an insoluble solid (zinc oxide) from a liquid (zinc chloride solution).
(a)(iii) Heat the solution to evaporate some water until saturation point is reached (1), then allow it to cool for crystals to form (1). Filter off the crystals (1) and dry them between filter papers (1).
The process involves concentrating the solution by evaporation, then allowing crystals to form through cooling. The crystals are then separated and dried.
(b) H+
All acids contain hydrogen ions (H+) in aqueous solution, which is what gives them their acidic properties.
(c)(i) A reaction that releases thermal energy/heat to the surroundings.
Exothermic reactions are characterized by energy being given out, usually in the form of heat.
(c)(ii) ZnO + 2HCl should be written on the higher energy line (left side), and ZnCl2 + H2O on the lower energy line (right side).
The reactants should be placed at a higher energy level than the products to show energy is released.
(c)(iii) The energy level of the reactants is higher than the energy level of the products, showing energy is released.
In an exothermic reaction, the products are at a lower energy level than the reactants, with the difference being released as heat.
(d) pH 2: red/pink (1), pH 12: blue (1)
Litmus is red in acidic solutions (pH < 7) and blue in alkaline solutions (pH > 7). At pH 2 (strongly acidic) it’s red, and at pH 12 (strongly alkaline) it’s blue.
Topic – 6.2
(a) A student investigates the reaction of small pieces of magnesium oxide with excess dilute hydrochloric acid of three different concentrations.
The time taken for each reaction to finish is recorded.
The three concentrations of the acid are:
- 0.4 mol/dm³
- 0.8 mol/dm³
- 1.6 mol/dm³
All other conditions stay the same.
Table 8.1 shows the time taken for each reaction to finish.
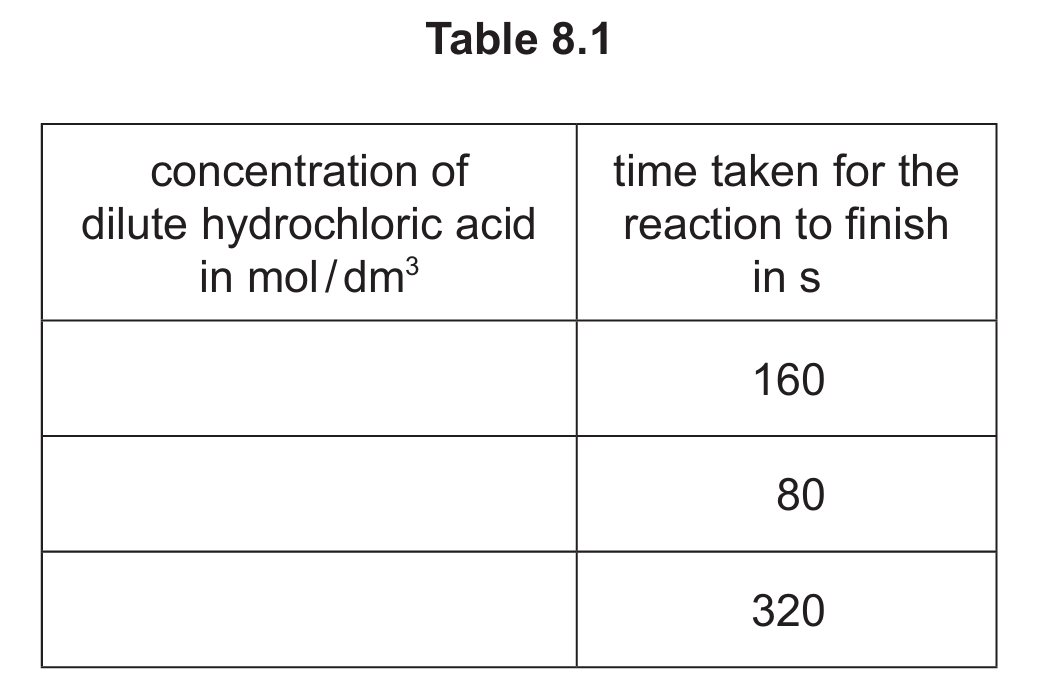
(i) Complete Table 8.1 by writing the concentrations in the first column.
(ii) Describe the effect on the time taken for the reaction to finish when the reaction is carried out at a lower temperature. All other conditions stay the same.
(iii) Describe the effect on the time taken for the reaction to finish when large pieces of magnesium oxide are used instead of small pieces of magnesium oxide. All other conditions stay the same.
(b) Molten magnesium chloride is electrolysed using inert electrodes.
(i) Name the products at the positive and negative electrodes.
(ii) Describe the arrangement, motion and separation of the particles in liquid magnesium chloride.
▶️ Answer/Explanation
(a)(i) The completed table should be:
- 1.6 mol/dm³ – 80 s
- 0.8 mol/dm³ – 160 s
- 0.4 mol/dm³ – 320 s
This shows that higher concentration leads to faster reaction (shorter time). The most concentrated acid (1.6 mol/dm³) reacts fastest (80 s), while the least concentrated (0.4 mol/dm³) reacts slowest (320 s).
(a)(ii) At a lower temperature, the time taken for the reaction to finish would increase (the reaction would be slower).
This is because at lower temperatures, particles have less kinetic energy, resulting in fewer successful collisions per unit time between the magnesium oxide and hydrochloric acid particles.
(a)(iii) Using large pieces instead of small pieces would increase the time taken for the reaction to finish (the reaction would be slower).
Larger pieces have less surface area exposed to the acid compared to the same mass of smaller pieces, reducing the frequency of collisions between reactant particles.
(b)(i) Products at electrodes:
- Positive electrode (anode): Chlorine gas (Cl₂)
- Negative electrode (cathode): Magnesium metal (Mg)
This occurs because in molten MgCl₂, Mg²⁺ ions are attracted to the cathode where they gain electrons to form Mg metal, while Cl⁻ ions are attracted to the anode where they lose electrons to form Cl₂ gas.
(b)(ii) In liquid magnesium chloride:
- Arrangement: The particles (Mg²⁺ and Cl⁻ ions) have an irregular arrangement with no long-range order.
- Motion: The ions can slide over one another, allowing the liquid to flow, but they remain in close contact.
- Separation: The ions are close together but not in fixed positions as in a solid, allowing some movement while maintaining the liquid state.
This describes the typical behavior of ionic compounds in the liquid state, where the electrostatic forces between ions are still strong but the ions have enough energy to move past one another.
