Topic – 2.1
A list of substances is shown:
bauxite, carbon dioxide, cryolite, ethane, ethanol, ethene, graphite, helium, hematite, hydrogen, silicon(IV) oxide, sodium chloride
Answer the following questions using only the substances from the list. Each substance may be used once, more than once or not at all.
State which substance:
(a) is manufactured by fermentation
(b) is monatomic
(c) is a reactant in photosynthesis
(d) is a solvent in the extraction of aluminium
(e) is an ore of iron
(f) is manufactured from methane
(g) is a compound with a giant covalent structure
(h) is used as a lubricant
(i) is tested for with limewater
▶️ Answer/Explanation
(a) ethanol or carbon dioxide
Ethanol is produced by fermentation of sugars, and carbon dioxide is a byproduct of this process.
(b) helium
Helium is a noble gas that exists as single atoms (monatomic), unlike other gases which are diatomic (e.g., H₂, O₂).
(c) carbon dioxide
In photosynthesis, plants use carbon dioxide and water to produce glucose and oxygen in the presence of sunlight.
(d) cryolite
Cryolite (Na₃AlF₆) is used as a solvent in the extraction of aluminium from bauxite to lower the melting point of the mixture.
(e) hematite
Hematite (Fe₂O₃) is one of the main ores of iron used in iron production.
(f) hydrogen
Hydrogen can be manufactured from methane through steam reforming or other chemical processes.
(g) silicon(IV) oxide
Silicon(IV) oxide (SiO₂) has a giant covalent structure where each silicon atom is covalently bonded to four oxygen atoms.
(h) graphite
Graphite is used as a lubricant because its layered structure allows the layers to slide over each other easily.
(i) carbon dioxide
Carbon dioxide is tested with limewater (calcium hydroxide solution), which turns milky/cloudy in its presence due to formation of calcium carbonate.
Topic – 4.1
This question is about electrolysis.
(a) State the meaning of the term electrolysis.
(b) Table 2.1 gives some information about the electrolysis of two electrolytes using graphite electrodes.
Table 2.1
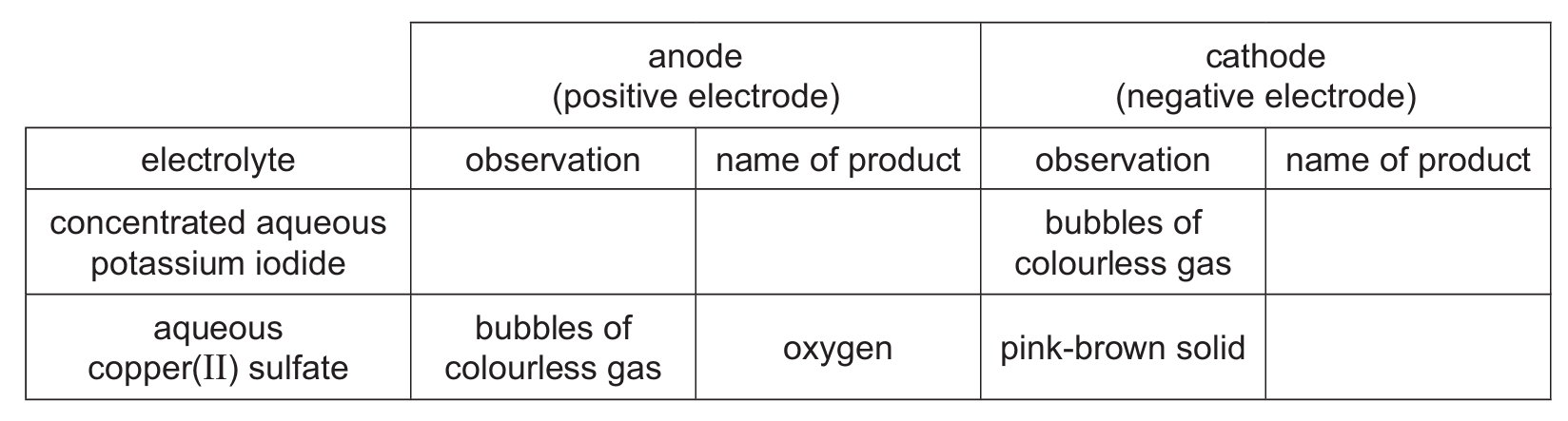
(i) Complete Table 2.1.
(ii) Oxygen is produced at the anode by the electrolysis of aqueous copper(II) sulfate. Write the ionic half-equation for this reaction.
(c) Aqueous copper(II) sulfate is electrolysed using copper electrodes instead of graphite electrodes.
(i) Explain why the mass of the anode decreases during this electrolysis.
(ii) Name the product formed at the cathode.
(iii) State what change, if any, is observed in the appearance of the aqueous copper(II) sulfate.
▶️ Answer/Explanation
(a) Electrolysis is the breakdown of an ionic compound in molten or aqueous state by the passage of electricity.
(b)(i) For concentrated aqueous potassium iodide:
Anode product: iodine (brown solution or black solid)
Cathode product: hydrogen (bubbles of colourless gas)
For aqueous copper(II) sulfate:
Cathode product: copper (pink-brown solid)
(b)(ii) The ionic half-equation for oxygen production at the anode is:
\(4OH^- \rightarrow 2H_2O + O_2 + 4e^-\)
(c)(i) The mass of the anode decreases because copper atoms from the anode lose electrons to form copper ions (\(Cu^{2+}\)) which go into the solution.
(c)(ii) The product formed at the cathode is copper.
(c)(iii) There is no change in the appearance of the aqueous copper(II) sulfate solution.
Detailed Explanation:
1. Electrolysis definition: Electrolysis involves the decomposition of ionic compounds when electricity is passed through them in molten or aqueous state. The ions move to the oppositely charged electrodes where they gain or lose electrons.
2. Electrolysis of potassium iodide:
– At the anode: Iodide ions (\(I^-\)) are oxidized to iodine (\(I_2\)), which appears as a brown solution or black solid.
– At the cathode: Hydrogen ions (\(H^+\)) from water are reduced to hydrogen gas (\(H_2\)), seen as bubbles.
3. Electrolysis of copper(II) sulfate:
– At the anode: Hydroxide ions (\(OH^-\)) from water are oxidized to oxygen gas (\(O_2\)) and water.
– At the cathode: Copper ions (\(Cu^{2+}\)) are reduced to copper metal, forming a pink-brown deposit.
4. Using copper electrodes:
– The copper anode dissolves as copper atoms lose electrons to become \(Cu^{2+}\) ions.
– At the cathode, these \(Cu^{2+}\) ions are reduced back to copper metal.
– The concentration of copper ions in solution remains constant, so no color change occurs.
Topic – 2.4
This question is about compounds of tin.
(a) Tin(IV) oxide has the formula SnO₂. The relative formula mass, \( M_r \) of SnO₂ is 151. Calculate the percentage by mass of tin in SnO₂.
(b) SnO₂ is an amphoteric oxide. SnO₂ reacts with aqueous sodium hydroxide, NaOH, to form a sodium salt and water only. The sodium salt contains a negative ion with the formula SnO₃²⁻.
(i) State the meaning of the term amphoteric.
(ii) Write the symbol equation for the reaction between SnO₂ and NaOH.
(c) Tin is a metal that forms both covalent and ionic compounds. Suggest why this is unusual for a metal.
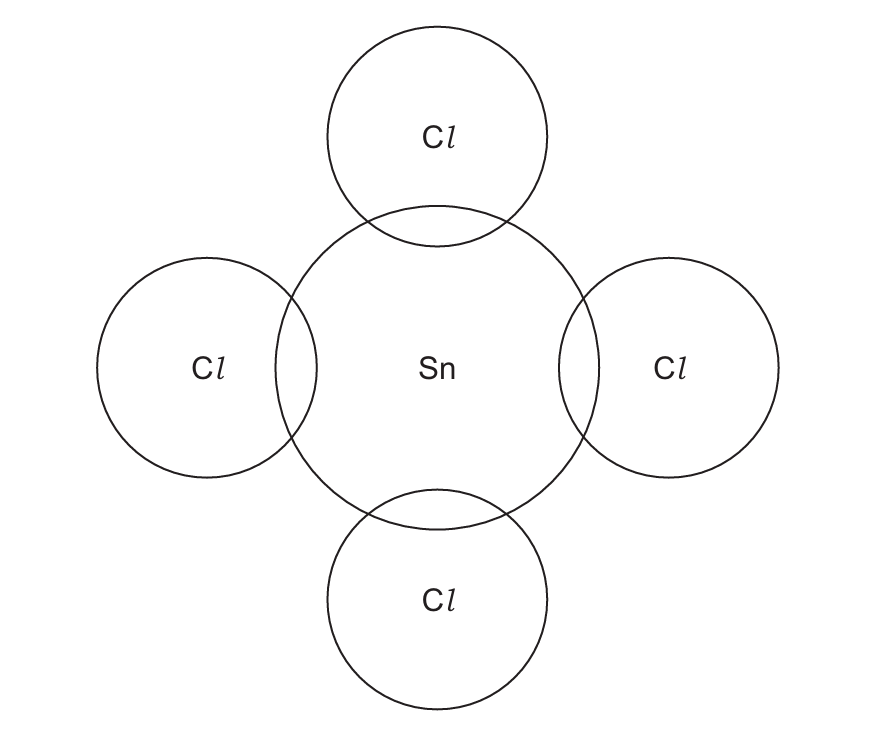
(d) (i) Tin(IV) chloride, SnCl₄ is covalently bonded. A tin atom has four electrons in its outer shell. Complete the dot-and-cross diagram in Fig. 3.1 for a molecule of SnCl₄. Show the outer shell electrons only.
(ii) Tin(II) oxide, SnO, is ionically bonded. The melting points of SnCl₄ and SnO are shown in Table 3.1.
Table 3.1

Explain, in terms of structure and bonding, why SnCl₄ has a much lower melting point than SnO.
(e) Part of the reactivity series is shown.
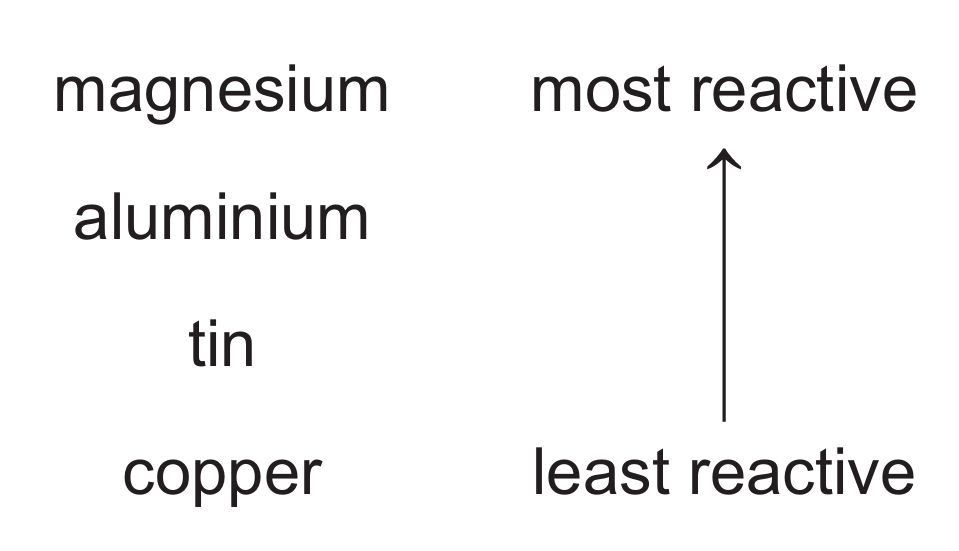
(i) When aluminium foil is added to aqueous tin(II) sulfate, a reaction does not occur even though aluminium is above tin in the reactivity series. Explain why a reaction does not occur.
(ii) An aqueous solution of tin(II) sulfate contains Sn²⁺ ions. Two experiments are carried out.
Experiment 1 Copper is added to aqueous tin(II) sulfate.
Experiment 2 Magnesium is added to aqueous tin(II) sulfate.
Write an ionic equation for any reaction that occurs in each experiment. If no reaction occurs, write ‘no reaction’.
(f) Hydrated tin(II) nitrate, Sn(NO₃)₂•20H₂O, decomposes when it is heated.
(i) State what is meant by the term hydrated.
(ii) Complete the equation for the decomposition of Sn(NO₃)₂•20H₂O.
2Sn(NO₃)₂•20H₂O → ……SnO + ……NO₂ + O₂ + ……H₂O
▶️ Answer/Explanation
(a) 78.8%
Calculation: Relative atomic mass of Sn = 119, O = 16. Percentage of Sn = (119/151) × 100 = 78.8%.
(b)(i) Reacts with acids and with bases to produce a salt and water.
(b)(ii) \( \text{SnO}_2 + 2\text{NaOH} \rightarrow \text{Na}_2\text{SnO}_3 + \text{H}_2\text{O} \)
The reaction shows tin(IV) oxide reacting with sodium hydroxide to form sodium stannate (Na₂SnO₃) and water.
(c) Metals typically form ionic compounds only, while covalent compounds usually contain non-metals only. Tin’s ability to form both is unusual.
(d)(i) The dot-and-cross diagram should show:
- Four single bonds between tin and chlorine atoms
- Three lone pairs on each chlorine atom
- No lone pairs on the tin atom
(d)(ii)
- SnCl₄ has simple molecular structure with weak intermolecular forces between molecules
- SnO has giant ionic structure with strong electrostatic forces between ions
- Much more energy is needed to overcome the strong ionic bonds in SnO than the weak intermolecular forces in SnCl₄
(e)(i) Aluminium forms an unreactive coating of aluminium oxide that prevents reaction.
(e)(ii)
- Experiment 1: no reaction (copper is below tin in reactivity series)
- Experiment 2: \( \text{Mg} + \text{Sn}^{2+} \rightarrow \text{Mg}^{2+} + \text{Sn} \)
(f)(i) A substance that is chemically combined with water or contains water of crystallization.
(f)(ii) \( 2\text{Sn(NO}_3\text{)}_2•20\text{H}_2\text{O} \rightarrow 2\text{SnO} + 4\text{NO}_2 + \text{O}_2 + 40\text{H}_2\text{O} \)
Balancing: For every 2 formula units of the hydrated salt, we get 2 SnO, 4 NO₂ (from 4 nitrate groups), 1 O₂ (remaining oxygen), and 40 H₂O (from 20 × 2 water molecules).
Topic – 7.3
This question is about sulfuric acid, \( H_2SO_4 \).
(a) Dilute sulfuric acid and aqueous sodium hydroxide can be used to prepare sodium sulfate crystals using a method that involves titration.
The apparatus for titration is shown in Fig. 4.1.

Thymolphthalein is used as an indicator for this titration.
(i) State the colour change of thymolphthalein at the end-point of this titration.
(ii) Suggest why universal indicator is not used for this titration.
(b) 25.0 cm³ of aqueous sodium hydroxide, NaOH, of concentration 0.100 mol/dm³ is neutralised by 20.0 cm³ of dilute sulfuric acid, \( H_2SO_4 \).
The equation for the reaction is shown.
\[2NaOH + H_2SO_4 \rightarrow Na_2SO_4 + 2H_2O\]
Calculate the concentration of \( H_2SO_4 \) using the following steps.
– Calculate the number of moles of NaOH used.
– Determine the number of moles of \( H_2SO_4 \) that react with the NaOH.
– Calculate the concentration of \( H_2SO_4 \).
(c) A student is provided with an aqueous solution of sodium sulfate.
Describe how to prepare a pure sample of sodium sulfate crystals from this solution.
(d) Potassium hydrogen sulfate, \( KHSO_4 \), can be prepared by a reaction between aqueous potassium hydroxide and dilute sulfuric acid. Water is the only other product.
Write a symbol equation for this reaction.
(e) Potassium hydrogen sulfate, \( KHSO_4 \), dissolves in water to form solution X.
Solution X contains \( K^+ \), \( H^+ \) and \( SO_4^{2-} \) ions.
(i) Name the type of solution that contains \( H^+ \) ions.
(ii) State the observations when the following tests are done.
● A flame test is carried out on X.
● Solid copper(II) carbonate is added to X.
● Aqueous barium nitrate acidified with dilute nitric acid is added to X.
(f) 0.325 g of Zn is added to dilute sulfuric acid which contains 0.0100 moles of \( H_2SO_4 \).
The equation for this reaction is shown.
\[Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2\]
(i) Determine whether Zn or \( H_2SO_4 \) is the limiting reactant.
Explain your answer.
(ii) In another experiment, 48.0 cm³ of hydrogen gas, \( H_2 \), is produced. The experiment is carried out at room temperature and pressure, r.t.p.
Calculate the number of molecules in 48.0 cm³ of \( H_2 \) gas measured at r.t.p.
The value of the Avogadro constant is \( 6.02 \times 10^{23} \).
▶️ Answer/Explanation
(a)(i) from blue to colourless
Thymolphthalein is blue in basic solutions (pH > 9.3) and colorless in acidic solutions (pH < 8.0). The end-point occurs when the solution changes from basic to acidic.
(a)(ii) universal indicator has too many colour changes
Universal indicator shows a range of colors for different pH values, making it difficult to identify the exact end-point of the titration.
(b)
Step 1: Moles of NaOH = concentration × volume = 0.100 mol/dm³ × 0.025 dm³ = 0.0025 mol
Step 2: From the equation, 2 moles NaOH react with 1 mole \( H_2SO_4 \), so moles \( H_2SO_4 \) = 0.0025 ÷ 2 = 0.00125 mol
Step 3: Concentration \( H_2SO_4 \) = moles ÷ volume = 0.00125 mol ÷ 0.020 dm³ = 0.0625 mol/dm³
(c)
1. Heat the solution to evaporate water until saturation point is reached (when crystals start to form)
2. Allow the solution to cool slowly, allowing crystals to form
3. Filter the crystals and dry them between filter papers or in a warm oven
(d) \( KOH + H_2SO_4 \rightarrow KHSO_4 + H_2O \)
(e)(i) acid / acidic solution
(e)(ii)
● Flame test: lilac flame (characteristic of potassium ions)
● Copper(II) carbonate added: solid dissolves, bubbles/fizzing observed (from \( CO_2 \) gas), and solution turns blue (from \( Cu^{2+} \) ions)
● Barium nitrate added: white precipitate forms (barium sulfate)
(f)(i)
Moles of Zn = mass ÷ molar mass = 0.325 g ÷ 65 g/mol = 0.005 mol
From the equation, 1 mole Zn reacts with 1 mole \( H_2SO_4 \). Since there are 0.0100 moles \( H_2SO_4 \) and only 0.005 moles Zn, Zn is the limiting reactant.
(f)(ii)
At r.t.p., 1 mole of gas occupies 24 dm³ (24,000 cm³)
Moles of \( H_2 \) = 48.0 cm³ ÷ 24,000 cm³/mol = 0.002 mol
Number of molecules = moles × Avogadro’s constant = 0.002 × 6.02 × 10²³ = 1.20 × 10²¹ molecules
Topic – 6.2
This question is about rate of reaction and equilibrium.
A student investigates the rate of decomposition of aqueous hydrogen peroxide, \( H_2O_2 \), using manganese(IV) oxide as a catalyst.
The equation for the reaction is shown.
\( 2H_2O_2(aq) \rightarrow 2H_2O(l) + O_2(g) \)
The student uses the apparatus shown in Fig. 5.1.
Fig. 5.1
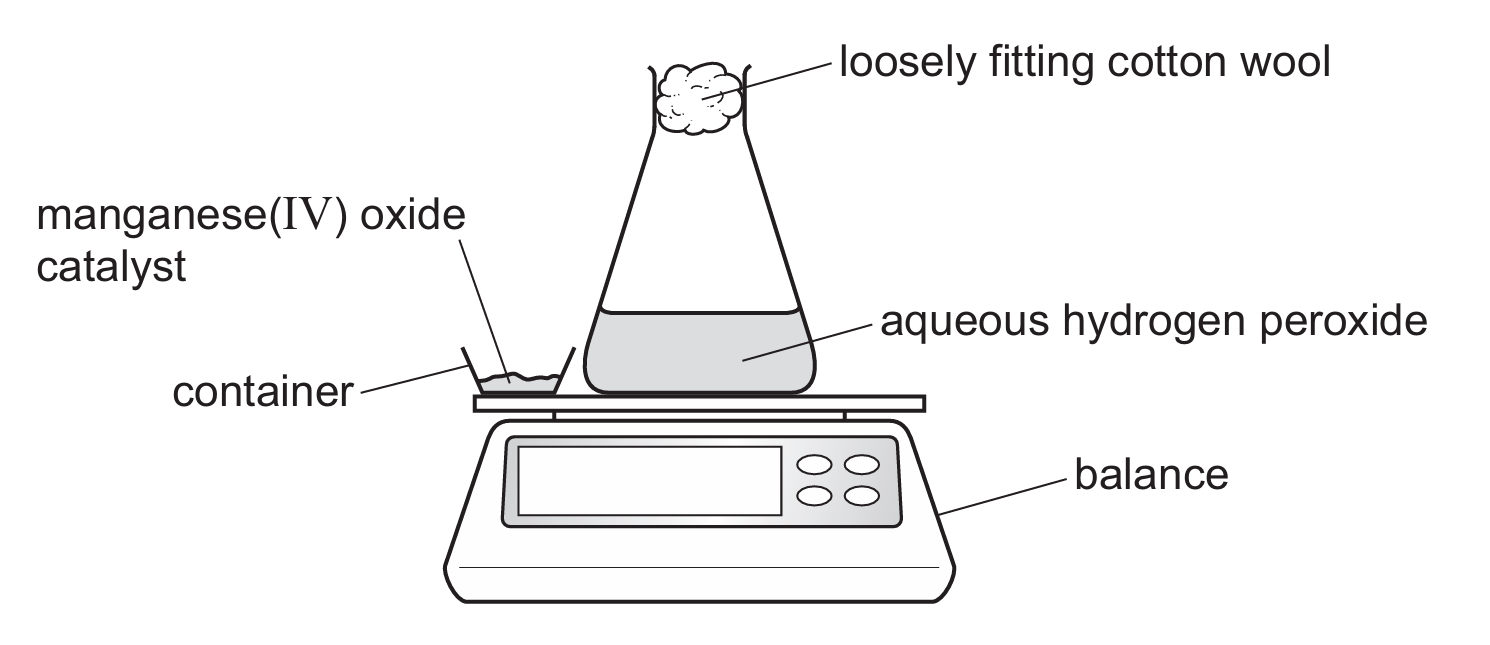
The student:
• adds the catalyst to the aqueous hydrogen peroxide
• replaces the container on the balance
• starts a stop-watch
• records the mass at regular time intervals.
(a) Table 5.1 shows the mass recorded at regular time intervals.
Table 5.1
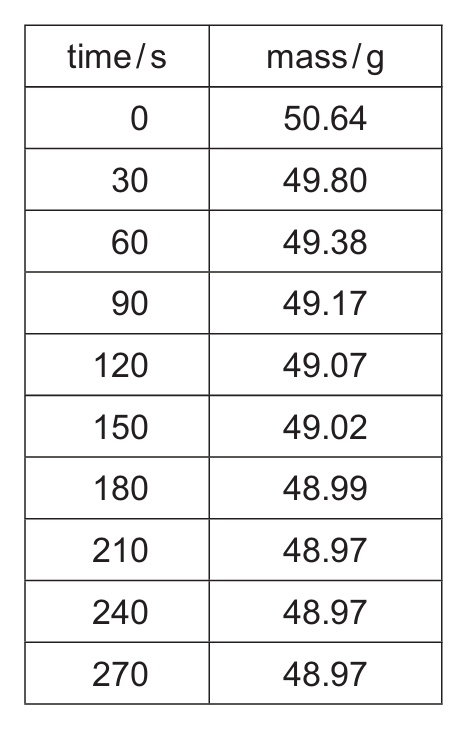
(i) Suggest why the mass decreases as time increases.
(ii) After a certain time the reaction stops. Explain why the reaction stops.
(iii) Suggest why it is not possible to use the results in Table 5.1 to determine the exact time when the reaction stops.
(b) Fig. 5.2 shows a graph of the mass against time.
Fig. 5.2
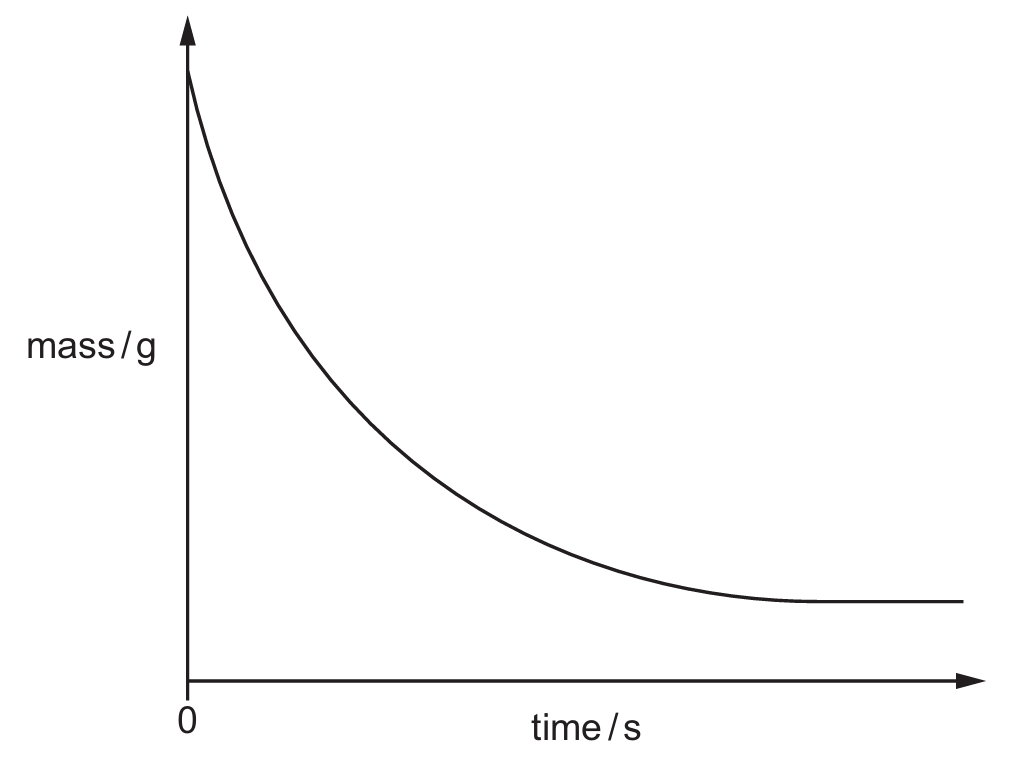
The experiment is repeated at a higher temperature. All other conditions remain the same.
(i) Explain, in terms of collision theory, why the rate of reaction is higher at a higher temperature.
(ii) On Fig. 5.2, sketch the line expected when the experiment is repeated at a higher temperature.
(c) Manganese(IV) oxide is the catalyst in this reaction.
(i) Explain the meaning of (IV) in manganese(IV) oxide.
(ii) State how the mass of the catalyst has changed, if at all, at the end of the experiment.
(d) Nitrogen monoxide gas, \( NO \), and oxygen gas, \( O_2 \), react to produce nitrogen dioxide gas, \( NO_2 \), at room temperature.
The reaction can reach equilibrium. The equation is shown.
\( 2NO(g) + O_2(g) \rightleftharpoons 2NO_2(g) \) \( \Delta H = -113 \, \text{kJ/mol} \)
NO(g) and \( O_2(g) \) are passed into a beaker as shown in Fig. 5.3.
Fig. 5.3
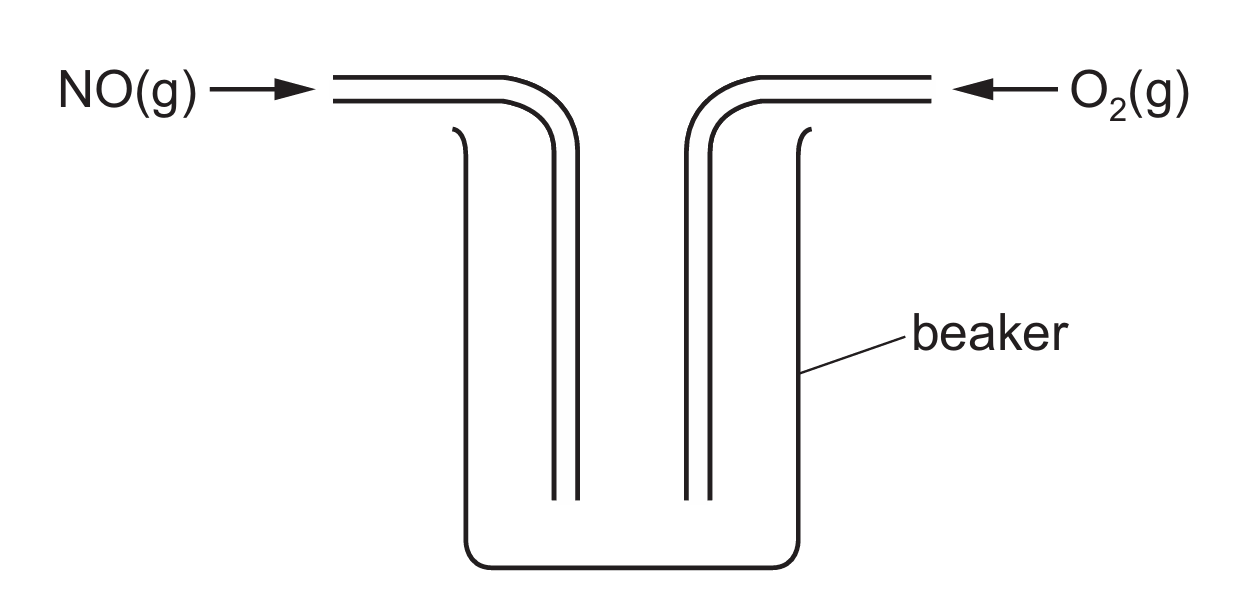
(i) Explain why the method shown in Fig. 5.3 will not allow the reaction to reach equilibrium.
(ii) The apparatus is changed and equilibrium is reached. The temperature of the equilibrium system is then increased and the position of equilibrium shifts to the left. Explain why the position of equilibrium shifts to the left.
(iii) The pressure of the equilibrium system is then increased. State the direction, if any, in which the position of equilibrium shifts. Explain your answer.
▶️ Answer/Explanation
(a)(i) Oxygen gas escapes from the flask.
The decomposition of hydrogen peroxide produces oxygen gas, which is allowed to escape through the loosely fitting cotton wool. As the gas escapes, the mass of the system decreases.
(a)(ii) The hydrogen peroxide is used up.
All the hydrogen peroxide has reacted or decomposed. Since the reaction requires hydrogen peroxide as a reactant, when it’s completely consumed, the reaction stops.
(a)(iii) Time intervals are too large.
The mass readings are taken at 30-second intervals, which may be too infrequent to pinpoint the exact moment when the reaction stops. The mass becomes constant between 210-270 seconds, but we can’t determine the exact time within this range.
(b)(i)
1. Kinetic energy of particles increases.
2. Frequency of collisions between particles increases.
3. More particles have energy greater than or equal to activation energy.
At higher temperatures, particles move faster and collide more frequently with greater energy, increasing the likelihood of successful collisions that lead to reaction.
(b)(ii)
1. Graph starts at same mass and has steeper gradient.
2. Levels off at the same mass but earlier.
The higher temperature causes a faster initial rate (steeper slope) but the reaction reaches completion sooner as reactants are used up more quickly.
(c)(i) Oxidation number of manganese is +4.
The Roman numeral (IV) indicates the oxidation state of manganese in this compound. Since oxygen typically has an oxidation state of -2, and the formula is MnO₂, the manganese must be +4 to balance the charges.
(c)(ii) No change.
Catalysts are not consumed in reactions. While manganese(IV) oxide participates in the reaction mechanism, it is regenerated and its mass remains unchanged at the end.
(d)(i) Not a closed system.
The beaker is open, allowing gases to escape. Equilibrium can only be established in a closed system where no reactants or products can enter or leave.
(d)(ii) Forward reaction is exothermic.
According to Le Chatelier’s principle, increasing temperature favors the endothermic direction. Since the forward reaction is exothermic (\(\Delta H\) negative), increasing temperature shifts equilibrium to the left (endothermic reverse reaction).
(d)(iii)
Direction: to the right.
Explanation: There are fewer gas molecules on the right (2 moles of NO₂) compared to the left (2 moles of NO + 1 mole of O₂ = 3 moles total). Increasing pressure shifts equilibrium to the side with fewer gas molecules.
Topic – 11.4
This question is about hydrocarbons.
(a) State the meaning of the term hydrocarbon.
(b) Propene, \( C_3H_6 \), can be made from long-chain alkanes such as dodecane. Dodecane contains 12 carbon atoms.
(i) Deduce the molecular formula of dodecane.
(ii) Name the type of reaction that occurs when long-chain alkanes are converted into shorter chain alkenes.
(c) Propene is an unsaturated hydrocarbon. Propene reacts with bromine.
(i) State the meaning of the term unsaturated.
(ii) Write the molecular formula of the product formed when propene reacts with bromine.
(d) A styrene molecule is represented as shown in Fig. 6.1.
Fig. 6.1
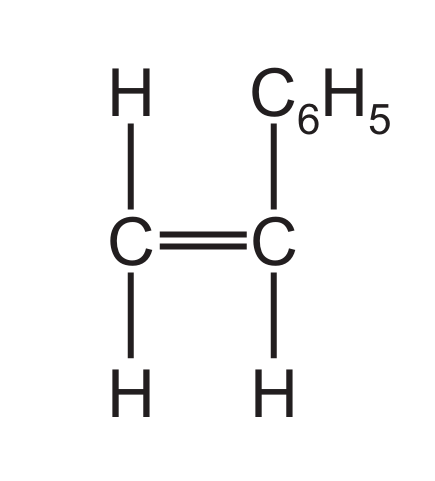
(i) The molecular formula of styrene is \( \text{C}_8\text{H}_8 \). Determine the empirical formula of styrene.
(ii) Styrene can be polymerised into poly(styrene). State the type of polymerisation that occurs when styrene is converted into poly(styrene).
(iii) Draw the structure of one repeat unit of poly(styrene). Include all of the atoms and all of the bonds. The \( \text{C}_8\text{H}_5 \) group should be represented as \( \text{C}_8\text{H}_5 \).
▶️ Answer/Explanation
(a) A compound containing carbon and hydrogen only.
(b)(i) \( C_{12}H_{26} \)
Alkanes have the general formula \( C_nH_{2n+2} \). For 12 carbon atoms (n=12), the number of hydrogen atoms would be \( 2(12)+2 = 26 \).
(b)(ii) Cracking
Cracking is the process where long-chain hydrocarbons are broken down into shorter, more useful molecules including alkenes.
(c)(i) Contains at least one carbon-carbon double bond.
Unsaturated hydrocarbons have double or triple bonds between carbon atoms, allowing them to undergo addition reactions.
(c)(ii) \( C_3H_6Br_2 \)
When propene (\( C_3H_6 \)) reacts with bromine (\( Br_2 \)), the double bond breaks and each carbon forms a bond with a bromine atom, resulting in 1,2-dibromopropane.
(d)(i) \( CH \)
The empirical formula is the simplest ratio of elements. For \( C_8H_8 \), the ratio is 1:1.
(d)(ii) Addition polymerisation
Styrene undergoes addition polymerisation where the double bond opens up to form bonds with adjacent molecules, creating a long polymer chain.
(d)(iii)
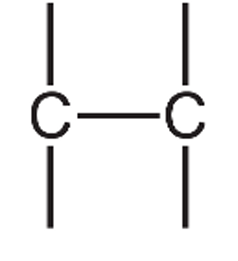
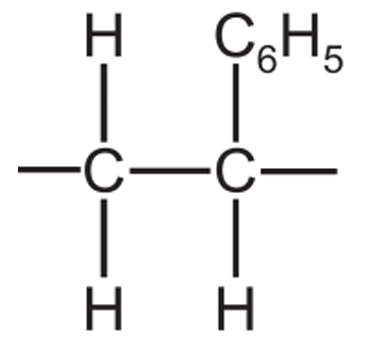
The repeat unit shows the opened double bond with extension bonds at each end, indicating where it connects to other repeat units in the polymer chain.
