Question 1:
Topic B1.1 (Characteristics of living organisms)
Which two characteristics of living organisms are demonstrated by gravitropism?
(A) growth and nutrition
(B) growth and sensitivity
(C) respiration and nutrition
(D) respiration and sensitivity
▶️Answer/Explanation
Ans: B
Question 2:
Topic B3.1 (Diffusion)
By which process does oxygen pass from the alveoli to the blood capillaries in the lungs?
(A) growth and nutrition
(B) growth and sensitivity
(C) respiration and nutrition
(D) respiration and sensitivity
▶️Answer/Explanation
Ans: A
Question 3:
Topic B7.3 (Digestion)
What are the products when oils are digested?
(A) amino acids and glycerol
(B) fats and amino acids
(C) fatty acids and glycerol
(D) fatty acids and sugars
▶️Answer/Explanation
Ans : C
Question 4:
Topic B7.2 (Digestive system)
The diagram shows part of the human alimentary canal and associated organs.

Which labels identify the liver, pancreas and stomach?

▶️Answer/Explanati
Ans: D
Question 5:
Topic B6.2 (Leaf structure)
The diagram shows the cross-section of part of a leaf with cells labelled W, X, Y and Z.

Which cells lose most water and which cells absorb most carbon dioxide during the daytime?
(A) W and X (B) X and Y (C) Y and Z (D) W and Z
▶️Answer/Explanation
Ans: A
Question 6:
Topic B5.1 (Enzymes)
A scientist places equal volumes of starch and saliva into a test-tube. After 30 minutes, the mixture in the test-tube is tested with iodine solution.
The iodine solution remains brown.
Which process does this experiment demonstrate?
(A) absorption
(B) assimilation
(C) digestion
(D) ingestion
▶️Answer/Explanation
Ans: C
Question 7:
Topic B9.4 (Blood)
The diagram shows some blood viewed under a light microscope.
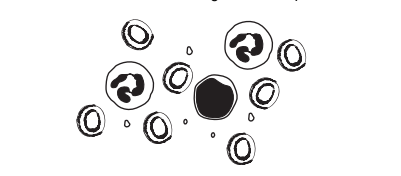
How many red blood cells are shown?
(A) 1 (B) 2 (C) 5 (D) 7
▶️Answer/Explanation
Ans: D
Question 8:
Topic B11.1 (Gas exchange in humans)
Which statement about the composition of expired air, compared with inspired air, is correct?
(A) The percentage of carbon dioxide is decreased and the percentage of water vapour is increased.
(B) The percentage of carbon dioxide is increased and the percentage of water vapour is increased.
(C) The percentage of oxygen is decreased and the percentage of carbon dioxide is decreased.
(D) The percentage of oxygen is increased and the percentage of carbon dioxide is decreased.
▶️Answer/Explanation
Ans: B
Question 9:
Topic B13.2 (Hormones)
Which row about hormones is correct?

▶️Answer/Explanation
Ans: C
Question 10:
Topic B15.4 (Sexual reproduction in humans)
The diagram shows the human female reproductive system.
Where is the embryo normally implanted to enable it to develop into a healthy fetus?

▶️Answer/Explanation
Ans: D
Question 11:
Topic B16.3 (Monohybrid inheritance)
Which statement describes a heterozygous genotype?
(A) not pure breeding with two different alleles
(B) not pure breeding with two identical alleles
(C) pure breeding with two different alleles
(D) pure breeding with two identical alleles
▶️Answer/Explanation
Ans: A
Question 12:
Topic B18.2 (Food chains and food webs)
Which statement about organisms in a food chain is correct?
(A) A carnivore is an organism that gets its energy by eating plants.
(B) A consumer is an organism that gets its energy by eating other organisms.
(C) A herbivore is an organism that gets its energy by eating animals.
(D) A producer is an organism that gets its energy from dead or waste matter.
▶️Answer/Explanation
Ans: B
Question 13:
Topic B18.3 (Carbon cycle)
The diagram shows gas exchange by two groups of organisms during the hours of darkness.

Which letters represent gases that are part of the carbon cycle?
(A) P and Q (B) P and R (C) P and S (D) Q and S
▶️Answer/Explanation
Ans: B
Question 14:
Topic C2.2 (Atomic structure and the Periodic Table)
Which statement about atom is correct?
(A) All atoms contain equal numbers of neutrons and protons.
(B) All atoms of the same element have the same number of neutrons.
(C) The Periodic Table lists atoms in order of increasing mass number.
(D) The smallest unit of an element is an atom.
▶️Answer/Explanation
Ans: D
Question 15:
Topic C2.5 (Simple molecules and covalent bonds)
Which substances exist as a covalent molecules?
1. helium
2. chlorine
3. sodium chloride
4. ethanol
(A) 1 and 2 (B) 1 and 3 (C) 2 and 4 (D) 3 and 4
▶️Answer/Explanation
Ans: C
Question 16:
Topic C2.6 (Giant covalent structures)
Which diagram shows the structure of graphite?
▶️Answer/Explanation
Ans: A
Question 17:
Topic C3.1 (Formulas)
Which oxides of nitrogen have the same ratio of nitrogen atoms to oxygen atoms?
1. N2O
2. NO
3. NO2
4. N2O4
(A) 1 and 2 (B) 1 and 3 (C) 2 and 4 (D) 3 and 4
▶️Answer/Explanation
Ans: D
Question 18:
Topic C6.2 (Rate of reaction)
Hydrogen gas is given off when zinc reacts with dilute sulfuric acid.
Which piece of apparatus is used to collect the hydrogen gas and measure its volume?
(A) balance
(B) gas syringe
(C) pipette
(D) test-tube
▶️Answer/Explanation
Ans: B
Question 19:
Topic C6.3 (Redox)
The equation for the reaction between iron(III) oxide, Fe2O3, and carbon is shown.
\( 2Fe_{2}O_{3} + 3C \to 4Fe +3 CO_{2}\)
Which statement about this reaction is correct?
(A) C is oxidised.
(B) CO2 is reduced.
(C) Fe is oxidised.
(D) Fe2O3 is oxidised.
▶️Answer/Explanation
Ans: A
Question 20:
Topic C7.1 (The characteristic properties of acids and bases)
The waste from a factory is acidic. The factory treats the waste with limestone.
Which row shows the pH of the waste before and after treatment?

▶️Answer/Explanation
Ans: B
Question 21:
Topic C12.5 (Identification of ions and gases)
White solid X reacts with dilute hydrochloric acid. A gas is produced which turns limewater milky. A flame test is done on solid X and produces a red coloured flame.
What is X?
(A) lithium carbonate
(B) lithium chloride
(C) potassium carbonate
(D) potassium chloride
▶️Answer/Explanation
Ans: A
Question 22:
Topic C2.1 (Elements, compounds and mixtures)
Different minerals contain different elements. Which mineral contains three non-metallic elements?
▶️Answer/Explanation
Ans: C
Question 23:
Topic C8.4 (Transition elements)
Manganese is a transition element. What is a property of manganese?
(A) high melting point
(B) low density
(C) thermal insulator
(D) transparent
▶️Answer/Explanation
Ans: A
Question 24:
Topic C9.3 (Alloys and their properties)
Which process is used to increase the hardness of a metal?
(A) Mix the metal with a gas.
(B) Mix the metal with another element.
(C) Mix the metal with a polymer.
(D) Mix the metal with its ore.
▶️Answer/Explanation
Ans: B
Question 25:
Topic C10.1 (Water)
Which compound contains two of the three elements needed in a fertiliser used for plant growth?
(A) potassium carbonate
(B) potassium chloride
(C) potassium nitrate
(D) potassium sulfate
▶️Answer/Explanation
Ans: C
Question 26:
Topic C6.1 (Physical and chemical changes)
Which type of reaction is used to manufacture lime from limestone?
(A) addition polymerisation
(B) cracking
(C) neutralisation
(D) thermal decomposition
▶️Answer/Explanation
Ans: D
Question 27:
Topic C11.7 (Polymers)
Poly(ethene) is made from ethene by addition polymerization.
Which word describes ethene in this process?
(A) fuel
(B) catalyst
(C) monomer
(D) solvent
▶️Answer/Explanation
Ans: C
Question 28:
Topic P1.2 (Motion)
The graph shows how the speed of an object varies with time.
The speed of the object is v at time t.

Which expression gives the distance travelled by the object in time t?
(A) \(\frac{1}{2}\left (\frac{v}{t} \right )\) (B) \(\frac{v}{t}\) (C) \(\frac{1}{2}\)vt (D) vt
▶️Answer/Explanation
Ans: C
Question 29:
Topic P1.4 (Density)
The diagram shows a block of metal of mass 72 g.

What is the density of the metal?
(A) 3.0 g/cm3 (B) 6.0 g/cm3 (C) 9.0 g/cm3 (D) 12 g/cm3
▶️Answer/Explanation
Ans: A
Question 30:
Topic P1.5.1 (Effects of forces)
Which statement about a resultant force is correct?
(A) An object must have a resultant force acting on it if it is moving.
(B) An object must have a resultant force acting on it if it is slowing down.
(C) Two forces must be in the same direction to produce a resultant force.
(D) Two forces must have the same magnitude to produce a resultant force.
▶️Answer/Explanation
Ans: B
Question 31:
Topic P1.6.3 (Energy resources)
What is the energy source for a wind turbine that is producing electricity?
(A) chemical potential energy of wind
(B) gravitational potential energy of wind
(C) kinetic energy of wind
(D) thermal energy of wind
▶️Answer/Explanation
Ans: C
Question 32:
Topic P2.2.1 (Thermal expansion of solids, liquids and gases)
A copper disc has a hole at its center that is slightly too small to fit over a copper cylinder.

How can the disc be fitted over the cylinder?
(A) Cool the disc and then fit it over the cylinder.
(B) Cool the disc, heat the cylinder and then fit the disc over the cylinder.
(C) Heat the cylinder and then fit it through the hole in the disc.
(D) Heat the disc and then fit it over the cylinder.
▶️Answer/Explanation
Ans: D
Question 33:
Topic P3.2.2 (Refraction of light)
The diagram shows a ray of light travelling from glass into air.
Which labelled arrow shows the path of the light in the air?

▶️Answer/Explanation
Ans: D
Question 34:
Topic P3.4 (Sound)
The diagrams represent two different sound waves, P and Q, drawn to the same scale.

▶️Answer/Explanation
Ans: D
Question 35:
Topic P4.2.1 (Electrical charge)
When two different, uncharged, insulating materials are rubbed together, one becomes positively charged and the other becomes negatively charged.
What happens to cause the materials to become charged?

▶️Answer/Explanation
Ans: C
Question 36:
Topic P4.2.3 (Voltage)
For which quantities is the unit the volt?
(A) current and potential difference (p.d.)
(B) electromotive force (e.m.f.) and potential difference (p.d.)
(C) electromotive force (e.m.f.) and resistance
(D) potential difference (p.d.) and resistance
▶️Answer/Explanation
Ans: B
Question 37:
Topic P4.3.2 (Series and parallel circuits)
A 2.0Ω resistor and a 1.0Ω resistor are connected in series with a cell.
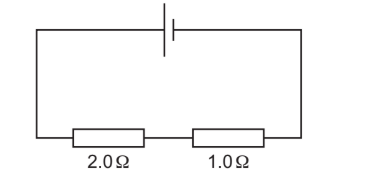
Which statement about current in the circuit is correct?
(A) The current in the 2.0Ω resistor is double the current in the 1.0Ω resistor.
(B) The current in the 2.0Ω resistor is equal to the current in the 1.0Ω resistor.
(C) The current in the 2.0Ω resistor is half the current in the 1.0Ω resistor.
(D) The current in the cell is larger than the current in either resistor.
▶️Answer/Explanation
Ans: B
Question 38:
Topic P4.5.5 (The d.c. motor)
A coil lies between the poles of a magnet. There is a current in the coil and this causes a turning effect.
Which change does not increase the turning effect on the coil?
(A) changing the direction of the current
(B) increasing the current
(C) using a stronger magnet
(D) using more turns in the coil
▶️Answer/Explanation
Ans: A
Question 39:
Topic P5.1 (The nucleus)
An atom of an isotope of strontium (Sr) has a proton number of 38 and contains 52 neutrons. What is the nuclide notation for this isotope?
(A) \(_{38}^{52}\textrm{Sr}\) (B) \(_{38}^{90}\textrm{Sr}\) (C) \(_{52}^{38}\textrm{Sr}\) (D) \(_{52}^{90}\textrm{Sr}\)
▶️Answer/Explanation
Ans: B
Question 40:
Topic P5.2.4 (Half-life)
The graph shows the decay curve for a radioactive substance.

What is the half-life of this substance?
(A) 2.0 hours
(B) 3.2 hours
(C) 5.0 hours
(D) 10 hours
▶️Answer/Explanation
Ans: A
