Question 1
(a) Fig. 1.1 is a diagram of the male reproductive system in humans.
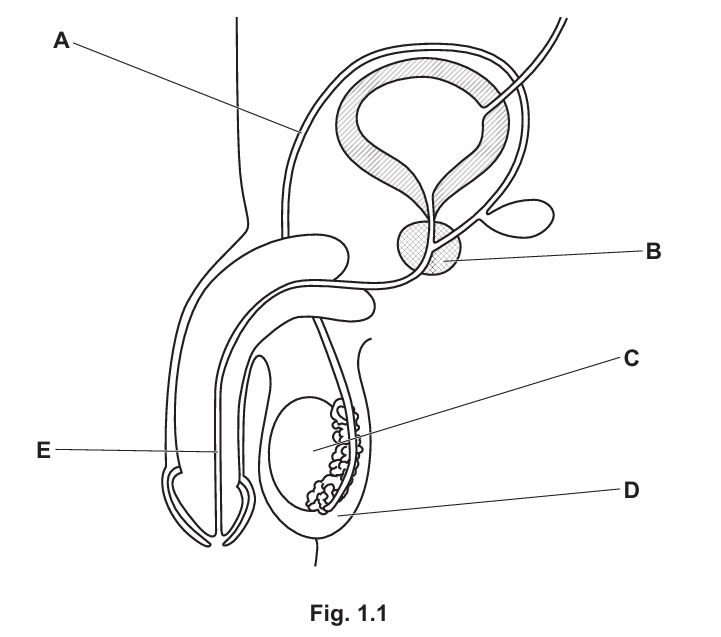
State the letter from Fig. 1.1 that represents the part: (Subtopic – B15.4)
- where meiosis occurs
- which secretes fluid for sperm to swim in
- which carries urine
- which produces sperm.
▶️Answer/Explanation
- where meiosis occurs: C
- which secretes fluid for sperm to swim in: B
- which carries urine: E
- which produces sperm: C
(b) Fig. 1.2 is a drawing of a sperm cell.
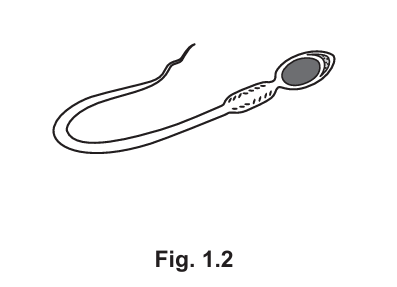
Label Fig. 1.2 to identify two features of sperm that adapt it for reproduction. (Subtopic – B15.4)
▶️Answer/Explanation
Two features of sperm that adapt it for reproduction are:
- Flagellum – for movement towards the egg.
- Haploid nucleus – contains half the genetic material needed for fertilization.
(c) Describe the difference between the arrangement of chromosomes found in the nuclei of sperm and those in a zygote. (Subtopic – B15.4)
▶️Answer/Explanation
The sperm contains a haploid nucleus with a single set of unpaired chromosomes, while the zygote contains a diploid nucleus with paired chromosomes (two sets of chromosomes).
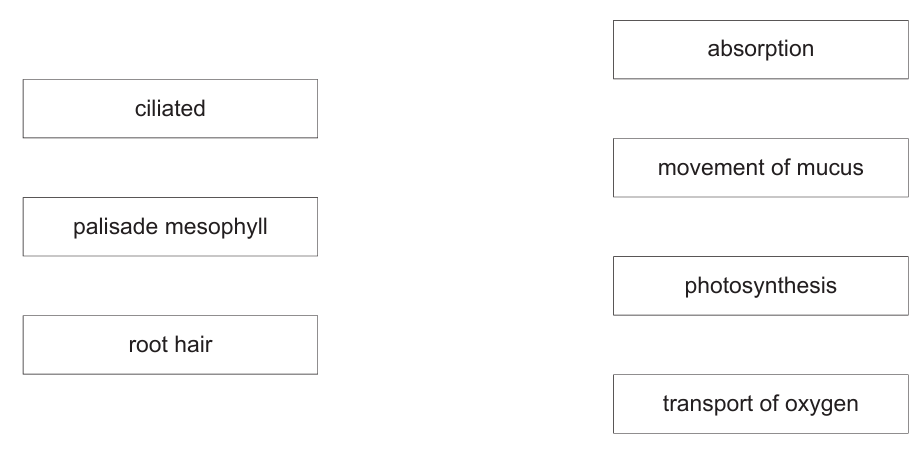
(d) Sperm and egg cells are specialised for their function of reproduction. The boxes on the left show some other specialised cells. The boxes on the right show some functions.
Draw lines to link each specialised cell with its function. (Subtopic – B2.1)
▶️Answer/Explanation
- ciliated – movement of mucus
- palisade mesophyll – photosynthesis
- root hair – absorption
Question 2
Alkanes are a type of hydrocarbon.
(a) State what is meant by a hydrocarbon. (Sub-topic – C11.1)
▶️Answer/Explanation
A hydrocarbon is a compound that contains only carbon and hydrogen atoms.
(b) Table 2.1 shows some information about some alkanes. ( Sub-topic – C11.4)
| Alkane | Molecular Formula | Energy Released when 1 g of Alkane is Completely Burned / kJ |
|---|---|---|
| Methane | CH4 | 55.6 |
| Butane | C4H10 | 51.7 |
| Octane | C8H18 | 48.0 |
| Eicosane | C20H42 | 46.4 |
The general formula for the alkanes is CnH2n+2, where n is the number of carbon atoms in the molecule.
(i) When n increases, the amount of energy released changes. State how the amount of energy released changes.
▶️Answer/Explanation
The amount of energy released decreases as n increases.
(ii) Tetradecane is an alkane with 14 carbon atoms. Write the molecular formula for tetradecane.
▶️Answer/Explanation
The molecular formula for tetradecane is C14H30.
(iii) Decene is not an alkane. It has the molecular formula C10H20. Use the general formula CnH2n+2 to show that decene is not an alkane.
▶️Answer/Explanation
For decene, n = 10. According to the general formula for alkanes, CnH2n+2, the number of hydrogen atoms should be 2(10) + 2 = 22. However, decene has only 20 hydrogen atoms, which means it does not follow the general formula for alkanes, hence it is not an alkane.
(c) Butane, C4H10, burns completely in air. Carbon dioxide and water are made. Construct the balanced symbol equation for this reaction. (Sub-topic – C6.2)
▶️Answer/Explanation
The balanced symbol equation for the complete combustion of butane is:
2C4H10 + 13O2 → 8CO2 + 10H2O
(d) (i) Burning butane is an exothermic reaction. State what is meant by an exothermic reaction. (Sub-topic – C5.1)
▶️Answer/Explanation
An exothermic reaction is a reaction that releases energy, usually in the form of heat, to the surroundings.
(ii) Use the axes shown in Fig. 2.1 to draw and label the energy level diagram for this reaction. Label:
- the energy of the reactants and the products
- the energy change in the reaction
- the activation energy of the reaction.
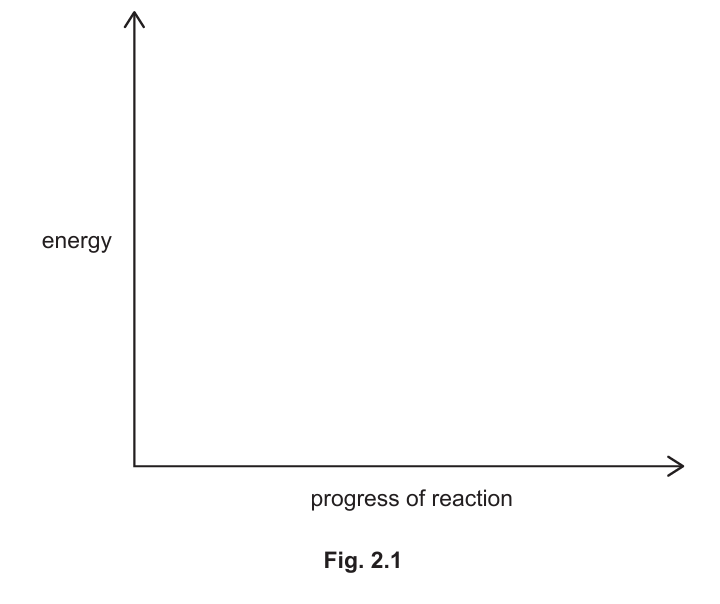
▶️Answer/Explanation
The energy level diagram should show:
- The reactants at a higher energy level than the products.
- The energy change (ΔH) as the difference between the energy of the reactants and the products.
- The activation energy (Ea) as the energy barrier that must be overcome for the reaction to proceed.
Here is a simplified representation:
Energy | | Reactants | /\ | / \ | / \ | / \ | / \ | / \ | / \ | / \ | / \ |/__________________\ Products
Question 3
Fig. 3.1 shows a crane lifting a wooden crate. (Sub-topic – P1.5.2)

(a) The crane is in equilibrium.
(i) The 1200 N counterweight is 5.0 m away from the pivot.
Calculate the moment of the counterweight about the pivot.
▶️Answer/Explanation
Answer: 6000 Nm
Explanation: The moment (M) is calculated using the formula \( M = F \times d \), where \( F \) is the force and \( d \) is the distance from the pivot. Here, \( F = 1200 \, \text{N} \) and \( d = 5.0 \, \text{m} \). Therefore, \( M = 1200 \times 5.0 = 6000 \, \text{Nm} \).
(ii) Determine the moment of the crate about the pivot.
▶️Answer/Explanation
Answer: 6000 Nm
Explanation: Since the crane is in equilibrium, the moment of the crate about the pivot must be equal to the moment of the counterweight. Therefore, the moment of the crate is also 6000 Nm.
(b) The crate gains 105 kJ of gravitational potential energy as it is lifted through a height of 42 m. (Sub-topic – P1.6.1)
Calculate the mass of the crate. The gravitational field strength, \( g \), is 10 N/kg.
▶️Answer/Explanation
Answer: 250 kg
Explanation: The gravitational potential energy (GPE) is given by the formula \( \text{GPE} = m \times g \times h \), where \( m \) is the mass, \( g \) is the gravitational field strength, and \( h \) is the height. Rearranging the formula to solve for mass, we get \( m = \frac{\text{GPE}}{g \times h} \). Substituting the given values, \( m = \frac{105000}{10 \times 42} = 250 \, \text{kg} \).
(c) The crane uses an electric motor. Fig. 3.2 shows a simple d.c. motor. (Sub-topic – P4.5.5)

(i) State the name of the component labelled Q in Fig. 3.2.
▶️Answer/Explanation
Answer: Split-ring commutator
Explanation: The component labelled Q is the split-ring commutator, which reverses the direction of current in the coil every half rotation, ensuring continuous rotation of the motor.
(ii) Draw an arrow on Fig. 3.2 to show the direction of the magnetic field.
▶️Answer/Explanation
Answer: Arrow drawn from N to S
Explanation: The magnetic field direction is from the North pole (N) to the South pole (S) of the magnet.
(iii) State two ways to increase the speed at which the coil rotates.
▶️Answer/Explanation
Answer:
- Increase the current
- Increase the magnetic field strength
Explanation: The speed of the coil rotation can be increased by increasing the current through the coil or by increasing the strength of the magnetic field. Both methods increase the force acting on the coil, resulting in faster rotation.
Question 4
(a) A seed germinates. State two environmental conditions needed for germination. ( Sub-topic – B15.3)
▶️Answer/Explanation
Answer:
1. Water/moisture
2. Oxygen
3. Suitable temperature/warmth
Explanation:
For a seed to germinate, it requires water to activate the metabolic processes, oxygen for respiration, and a suitable temperature to ensure the enzymes involved in germination function optimally.
(b) A plant is kept in the dark to grow. Fig. 4.1 shows the growth of the plant shoot. (Sub-topic – B13.1)
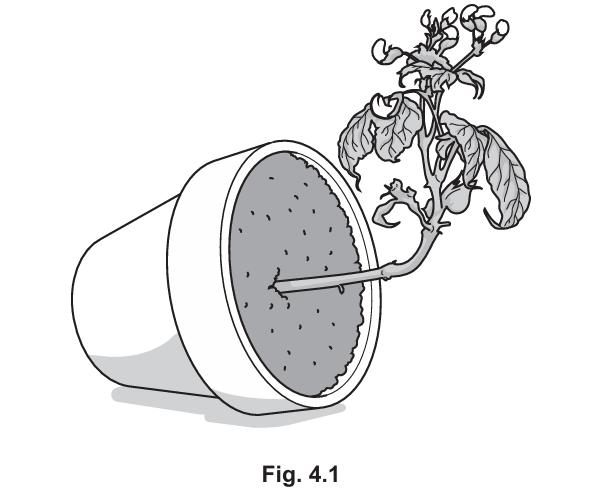
(i) State the name of the tropic response shown in Fig. 4.1.
▶️Answer/Explanation
Answer:
Gravitropism
Explanation:
Gravitropism is the growth response of a plant in relation to gravity. In the dark, the plant shoot grows upwards, away from the gravitational pull, which is an example of negative gravitropism.
(ii) Complete the sentences to explain the mechanism of this growth response.
A plant hormone called …… is made in the shoot tip and moves through the plant. The hormone collects on the …… side of the shoot. This stimulates growth causing cell …… The shoot grows away from the direction of ……
▶️Answer/Explanation
Answer:
A plant hormone called auxin is made in the shoot tip and moves through the plant. The hormone collects on the lower side of the shoot. This stimulates growth causing cell elongation. The shoot grows away from the direction of gravity.
Explanation:
Auxin is a plant hormone that promotes cell elongation. When a plant is kept in the dark, auxin accumulates on the lower side of the shoot due to gravity. This causes the cells on the lower side to elongate more than those on the upper side, resulting in the shoot growing upwards, away from gravity.
(c) Plants photosynthesize. (Sub-topic – B6.1)
(i) State the balanced symbol equation for photosynthesis.
▶️Answer/Explanation
Answer:
\(6CO_2 + 6H_2O \rightarrow C_6H_{12}O_6 + 6O_2\)
Explanation:
Photosynthesis is the process by which plants convert carbon dioxide and water into glucose and oxygen, using light energy. The balanced equation shows that six molecules of carbon dioxide and six molecules of water produce one molecule of glucose and six molecules of oxygen.
(ii) Explain why chlorophyll is needed for photosynthesis.
▶️Answer/Explanation
Answer:
Chlorophyll absorbs light energy, which is used to convert carbon dioxide and water into glucose and oxygen.
Explanation:
Chlorophyll is a green pigment found in the chloroplasts of plant cells. It absorbs light energy, primarily from the blue and red wavelengths, and uses this energy to drive the chemical reactions of photosynthesis. Without chlorophyll, plants would not be able to capture light energy and convert it into chemical energy in the form of glucose.
Question 5
(a) Fig. 5.1 shows the arrangement of particles in a liquid.
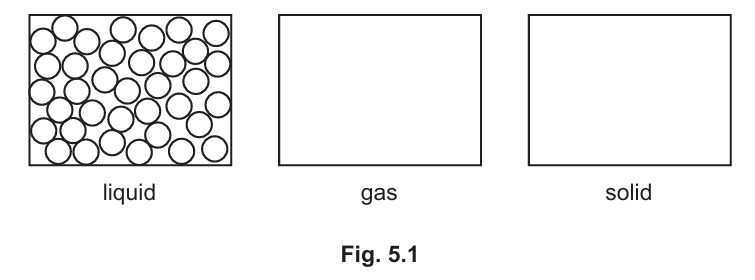
Complete Fig. 5.1 to show the arrangement of the particles in a gas and in a solid. (Sub-topic – C1.1)
▶️Answer/Explanation
Answer:
– Gas: Particles are far apart and move freely in all directions.
– Solid: Particles are closely packed in a regular pattern and vibrate in fixed positions.
Explanation: In a gas, particles have more energy and move randomly, while in a solid, particles are tightly packed and have less energy, causing them to vibrate in place.
(b)(i) Liquid water boils at 100°C to form steam. (Sub-topic – C1.1)
Describe what happens to the water particles during this change of state.
Include:
- how the arrangement of the particles changes
- how the movement of the particles changes.
▶️Answer/Explanation
Answer:
– Arrangement: The particles move further apart as the liquid turns into a gas.
– Movement: The particles move more freely and randomly in all directions.
Explanation: When water boils, the particles gain enough energy to overcome the forces holding them together in the liquid state, causing them to move further apart and more freely, forming a gas.
(b)(ii) Fig. 5.2 shows the bonds between the atoms and the forces between the molecules in water. (Sub-topic – C1.1)
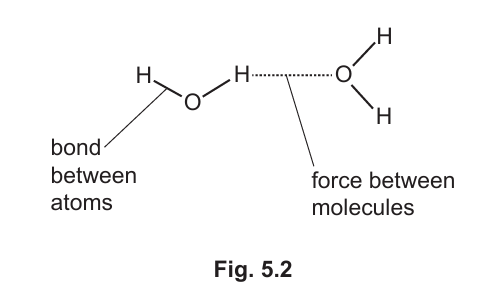
When water boils, the forces between the molecules are broken.
Explain why the bonds between atoms are not broken.
▶️Answer/Explanation
Answer:
The bonds between atoms (covalent bonds) are strong and require a lot of energy to break, whereas the forces between molecules (intermolecular forces) are weaker and can be broken with less energy.
Explanation: During boiling, only the weaker intermolecular forces are overcome, allowing the molecules to separate and form a gas. The covalent bonds within the water molecules remain intact because they require much more energy to break.
(b) (iii) Draw a dot-and-cross diagram to show the bonding in water, H2O. Show only the outer shell electrons.
▶️Answer/Explanation
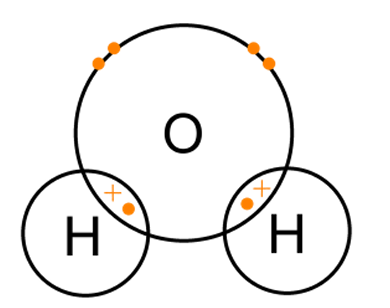
2 shared pairs ;
all else correct ;
(c)(i) Water reacts with magnesium metal. The reaction is very slow. (Sub-topic – C6.2)
The reaction is faster if hot water is used.
Explain why. Use ideas about collisions between particles.
▶️Answer/Explanation
Answer:
The reaction is faster with hot water because the particles have more kinetic energy, leading to more frequent and energetic collisions.
Explanation: At higher temperatures, particles move faster and collide more frequently with greater energy, increasing the likelihood of successful collisions that result in a reaction.
(ii) The reaction between water and magnesium is faster if powdered magnesium is used instead of strips of magnesium.
Explain why. Use ideas about collisions between particles.
▶️Answer/Explanation
more surface area ;
more frequent collisions / more collisions per second / owtte ;
Question 6
Fig. 6.1 shows a man paddling a canoe on a lake. The arrows show the horizontal forces acting on the canoe. (Sub-topic – P1.2)
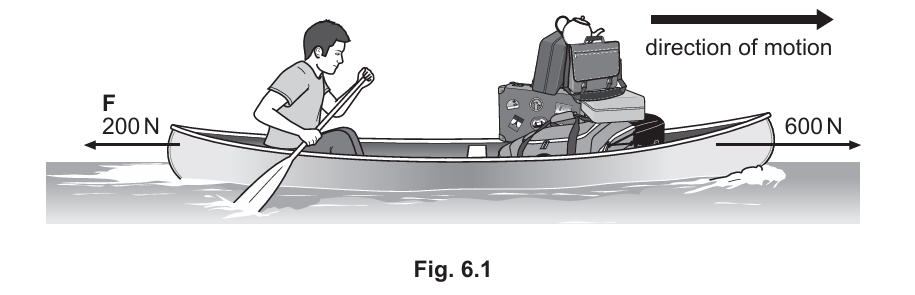
(a) (i) State the cause of the force labelled F on Fig. 6.1.
▶️Answer/Explanation
The force labelled F is caused by friction (or drag/air resistance/water resistance).
(ii) The combined mass of the man and the canoe and his luggage is 100 kg. Calculate the acceleration of the canoe.
▶️Answer/Explanation
To calculate the acceleration, we use Newton’s second law: \( F = ma \).
The resultant force is \( 400 \, \text{N} \).
The mass \( m = 100 \, \text{kg} \).
Therefore, the acceleration \( a = \frac{F}{m} = \frac{400}{100} = 4 \, \text{m/s}^2 \).
(b) (i) The man counts 15 wavefronts passing a point in 1 minute. Calculate the frequency of the waves in Hz.
▶️Answer/Explanation
Frequency is calculated as the number of wavefronts passing a point per second.
Number of wavefronts = 15.
Time = 1 minute = 60 seconds.
Frequency \( f = \frac{15}{60} = 0.25 \, \text{Hz} \).
(ii) The wavelength of the water waves is 0.6 m. Use your answer to 6(b)(i) to calculate the speed of the water waves.
▶️Answer/Explanation
The speed of the waves is calculated using the formula \( v = f \lambda \).
Frequency \( f = 0.25 \, \text{Hz} \).
Wavelength \( \lambda = 0.6 \, \text{m} \).
Therefore, the speed \( v = 0.25 \times 0.6 = 0.15 \, \text{m/s} \).
(iii) Fig. 6.2 shows the wavefronts of the water waves moving towards two rocks. The water waves will diffract as they travel between the two rocks.
Complete Fig. 6.2 to show how the water waves are diffracted.
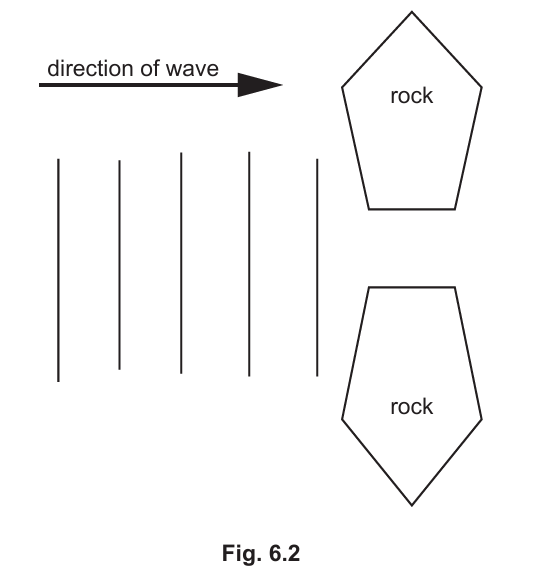
▶️Answer/Explanation
The wavefronts should be drawn as circular waves spreading out after passing through the gap between the rocks. This shows the diffraction of the waves.
(c) The man uses a solar panel to charge his mobile phone. The solar panel uses energy from the Sun to generate electricity. State the name of the process in the Sun that releases energy.
▶️Answer/Explanation
The process in the Sun that releases energy is nuclear fusion.
Question 7
(a) Fig. 7.1 is a photograph of a person with a deficiency disease that has affected their bone growth. (Sub-topic – B7.1)

(i) State the name of the vitamin the person in Fig. 7.1 is deficient in.
▶️Answer/Explanation
Answer: Vitamin D
Explanation: The person in Fig. 7.1 is suffering from a deficiency disease that affects bone growth, which is commonly caused by a lack of Vitamin D. Vitamin D is essential for calcium absorption and bone health.
(ii) Taking vitamin supplements can prevent the deficiency disease shown in Fig. 7.1. Suggest two other ways to prevent the effects seen in Fig. 7.1.
▶️Answer/Explanation
Answer: Exposure to sunlight and consuming foods high in Vitamin D.
Explanation: Vitamin D can be synthesized by the body when the skin is exposed to sunlight. Additionally, consuming foods rich in Vitamin D, such as fatty fish, fortified milk, and eggs, can help prevent deficiency.
(b) Kwashiorkor is a deficiency disease caused by a lack of protein in the diet. Table 7.1 shows the ages of children admitted to a hospital with kwashiorkor disease. (Sub-topic – B7.1)

(i) Use Table 7.1 to calculate the percentage of children of age 4 and under with kwashiorkor disease admitted to the hospital. Give your answer to the nearest whole number.
▶️Answer/Explanation
Answer: 93%
Explanation: The percentage of children aged 4 and under with kwashiorkor disease is calculated as (88 / 95) × 100 = 92.63%, which rounds to 93%.
(ii) The recommended daily intake of protein per kg of body mass for a child is greater than that of an adult. State why.
▶️Answer/Explanation
Answer: Children require more protein for growth and development.
Explanation: Children are still growing, so they need more protein to support the development of tissues, muscles, and organs compared to adults, whose growth has stabilized.
(c) State the name of one other deficiency disease caused by protein-energy malnutrition. (Sub-topic – B7.1)
▶️Answer/Explanation
Answer: Marasmus
Explanation: Marasmus is another deficiency disease caused by severe protein-energy malnutrition, characterized by extreme weight loss and muscle wasting.
(d) State the names of the four elements that all proteins contain. (Sub-topic – B4.1)
▶️Answer/Explanation
Answer: Carbon, Hydrogen, Oxygen, and Nitrogen
Explanation: Proteins are composed of amino acids, which contain the elements carbon (C), hydrogen (H), oxygen (O), and nitrogen (N).
(e) State the name of the enzyme that breaks down proteins. (Sub-topic – B5.1)
▶️Answer/Explanation
Answer: Protease
Explanation: Protease is the enzyme responsible for breaking down proteins into smaller peptides and amino acids during digestion.
Question 8 (Subtopic: C10.2)
Some cars use petrol as a fuel. Some cars use diesel as a fuel.
Table 8.1 shows the mass of pollutant made when 1 kg of petrol or 1 kg of diesel is burnt in a car engine.

(a) (i) Car A uses 5 kg of petrol fuel for a journey. Car B uses 8 kg of diesel fuel for the same journey. State which car, A or B, makes the most nitrogen monoxide. Explain your answer.
▶️Answer/Explanation
Car A makes the most nitrogen monoxide because it uses petrol, which produces more nitrogen monoxide per kg of fuel burned compared to diesel.
Explanation: Car A uses petrol, which produces 59 g of nitrogen monoxide per kg of fuel burned. Car B uses diesel, which produces 29 g of nitrogen monoxide per kg of fuel burned. Therefore, Car A produces 5 × 59 = 295 g of nitrogen monoxide, while Car B produces 8 × 29 = 232 g of nitrogen monoxide.
(a) (ii) The nitrogen monoxide, NO, made inside the car engine is removed by a catalytic converter. The nitrogen monoxide is turned into nitrogen gas and oxygen gas. Construct the balanced symbol equation for this reaction.
▶️Answer/Explanation
The balanced symbol equation for the reaction is 2NO → N2 + O2.
(a) (iii) Sulfur dioxide is a pollutant that causes acid rain. Sulfur dioxide is not removed from car emissions by a catalytic converter. Describe one way that emissions of sulfur dioxide by cars can be reduced.
▶️Answer/Explanation
Using low-sulfur fuel reduces the amount of sulfur dioxide produced during combustion, thereby reducing emissions.
8 (b) A petrol car makes 236 g of carbon monoxide gas when 1 kg of petrol is burnt. Calculate the volume occupied by 236 g of carbon monoxide gas. The molar gas volume at room temperature and pressure is 24 dm3. Show your working.
▶️Answer/Explanation
The volume occupied by 236 g of carbon monoxide gas is 202 dm3.
Explanation:
Molar mass of CO = 12 + 16 = 28 g/mol
Moles of CO = 236 g / 28 g/mol = 8.43 mol
Volume of CO = 8.43 mol × 24 dm3/mol = 202 dm3
8 (c) Sulfur dioxide is used in the manufacture of sulfuric acid in the Contact process.
2SO2(g) + O2(g) ⇌ 2SO3(g)
Describe two conditions used for this reversible reaction. (Sub-topic – C6.3)
▶️Answer/Explanation
Two conditions used for the reversible reaction in the Contact process are a temperature of around 450°C and the use of a vanadium(V) oxide catalyst.
Explanation:
1. Temperature: The reaction is carried out at a temperature of around 450°C.
2. Catalyst: A vanadium(V) oxide (V2O5) catalyst is used to increase the rate of reaction.
Question 9
A student investigates the effect of changing temperature on the current through a thermistor. The student connects a cell, an NTC thermistor, an ammeter and a switch in series.
(a) On Fig. 9.1, complete the circuit diagram to show the circuit used by the student. (Sub-topic – P4.3.1)
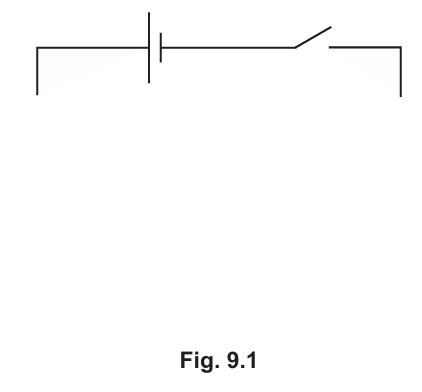
▶️Answer/Explanation
The circuit should include:
- A cell connected in series with the NTC thermistor, ammeter, and switch.
- The ammeter should be placed in series to measure the current through the thermistor.
- The switch should be placed in series to control the circuit.
(b) The graph in Fig. 9.2 shows the results obtained by the student.
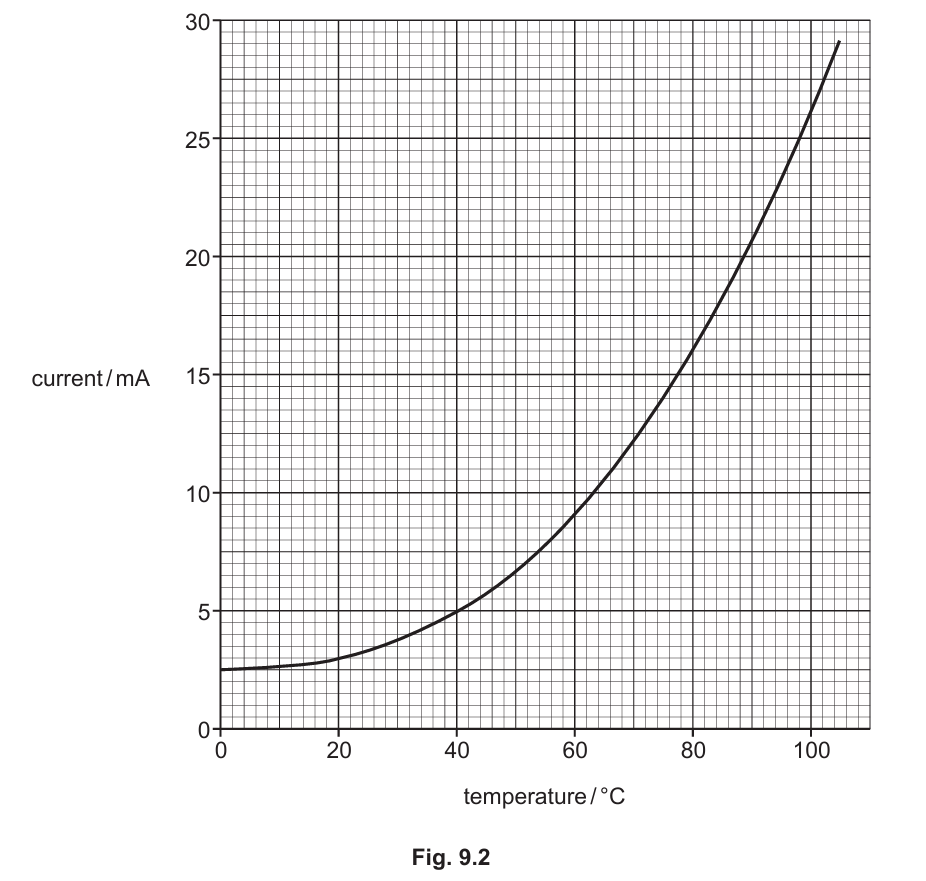
The temperature of the thermistor is 40°C. Calculate the time it takes for 1.0 C of charge to flow through the thermistor. (Sub-topic – P4.2.2)
▶️Answer/Explanation
To calculate the time, use the formula: \[ Q = I \times t \] Where:
- \( Q \) is the charge (1.0 C)
- \( I \) is the current (from the graph at 40°C)
- \( t \) is the time
From the graph, the current at 40°C is 5 mA (0.005 A). Therefore: \[ t = \frac{Q}{I} = \frac{1.0}{0.005} = 200 \text{ s} \] The time taken is 200 seconds.
(c) The student uses a liquid-in-glass thermometer to measure temperature. Fig. 9.3 shows the structure of a liquid-in-glass thermometer.
(i) Thermal energy is transferred through the glass to the ethanol. Describe how thermal energy is transferred through glass. (Sub-topic – P2.3.1)
▶️Answer/Explanation
Thermal energy is transferred through glass by conduction. In solids like glass, thermal energy is transferred by the vibration of particles. When one part of the glass is heated, the particles vibrate more vigorously and transfer this energy to neighboring particles, causing the thermal energy to move through the glass.
(ii) The ethanol in the thermometer expands as the temperature increases. Explain why the ethanol expands as the temperature increases in terms of the motion and arrangement of molecules. (Sub-topic – P2.2.2)
▶️Answer/Explanation
As the temperature increases, the kinetic energy of the ethanol molecules increases. This causes the molecules to move more rapidly and spread out, leading to an increase in volume. The increased motion and greater separation between molecules result in the expansion of the ethanol.
(iii) The volume of the ethanol in the thermometer at 25°C is 2.00 cm³ and the density of the ethanol is 0.78 g/cm³. When the thermometer is cooled to 3°C, the volume decreases to 1.95 cm³. Calculate the density of the ethanol at 3°C. (Sub-topic – P1.4)
▶️Answer/Explanation
First, calculate the mass of the ethanol using the initial volume and density: \[ \text{Mass} = \text{Density} \times \text{Volume} = 0.78 \times 2.00 = 1.56 \text{ g} \] Then, calculate the new density at 3°C using the new volume: \[ \text{Density} = \frac{\text{Mass}}{\text{Volume}} = \frac{1.56}{1.95} = 0.80 \text{ g/cm}^3 \] The density of the ethanol at 3°C is 0.80 g/cm³.
Question 10
(a) Fig. 10.1 is a diagram of a cross-section through skin. (Sub-topic – B13.3)
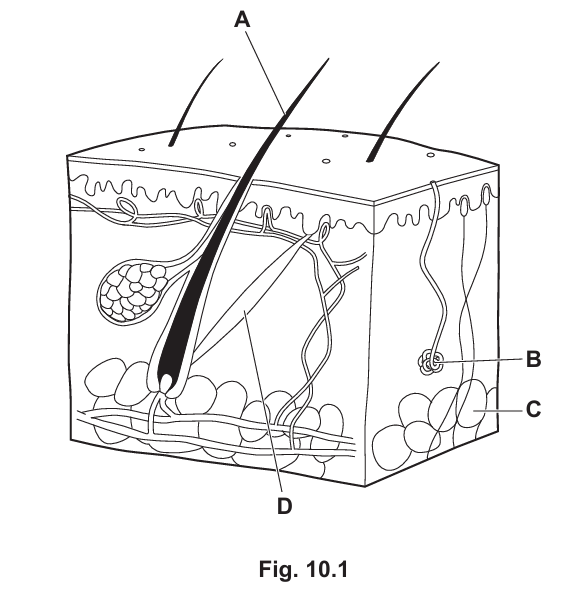
Table 10.1 shows the names and functions of some of the parts labelled A–D in Fig. 10.1.
Complete Table 10.1.
| Name of Part | Letter in Fig. 10.1 | Function |
|---|---|---|
| Provide insulation | ||
| Hair erector muscle | ||
| B |
▶️Answer/Explanation
Answer:
The table is completed as shown above. Fatty tissue (C) provides insulation, the hair erector muscle (D) contracts to raise hair, and the sweat gland (B) produces sweat.
| Name of Part | Letter in Fig. 10.1 | Function |
|---|---|---|
| Fatty tissue / fat | C | Provide insulation |
| Hair erector muscle | D | Contract to raise hair |
| Sweat gland | B | Produce sweat |
(b) Describe the role of arterioles in reducing body temperature when the body gets too hot. (Sub-topic – B13.3)
▶️Answer/Explanation
Answer:
When the body gets too hot, arterioles near the skin surface undergo vasodilation. This means they widen, allowing more blood to flow through the capillaries near the skin surface. As a result, more heat is lost from the blood to the environment through radiation, helping to cool the body down.
(c) The control of internal body temperature is an example of negative feedback. (Sub-topic – B13.3)
(i) Explain what is meant by the term negative feedback.
(ii) State one other example of negative feedback.
▶️Answer/Explanation
Answer:
(i) Negative feedback is a regulatory mechanism in which a change in a variable (e.g., body temperature) triggers a response that counteracts the initial change, bringing the variable back to its set point. For example, if body temperature rises, mechanisms like sweating and vasodilation are activated to cool the body down.
(ii) Another example of negative feedback is the regulation of blood glucose levels. When blood glucose levels rise, insulin is released to lower them, and when blood glucose levels fall, glucagon is released to raise them.
Question 11
(a) Copper oxide, CuO, is heated with carbon, C. Copper, Cu, and carbon dioxide, CO2, are made as shown in the equation:
2CuO + C → 2Cu + CO2
This reaction is an example of reduction. Use the equation to explain what reduction means. (Sub-topic – C6.3)
▶️Answer/Explanation
Reduction is the process in which a substance loses oxygen. In the given reaction, copper oxide (CuO) loses oxygen to form copper (Cu), which is why this reaction is an example of reduction.
(b) The copper made from copper oxide is not pure. A student purifies the impure copper using electrolysis. Fig. 11.1 shows the apparatus the student uses. (Sub-topic – C4.1)

(i) State the name of the electrolyte solution the student uses.
(ii) The student uses impure copper as the anode. State what the student uses as the cathode.
▶️Answer/Explanation
(i) The electrolyte solution used is copper(II) sulfate solution.
(ii) The student uses pure copper as the cathode.
(c) Copper atoms are formed from copper ions, Cu2+, at the cathode. Construct the balanced ionic half-equation for this reaction. Use the symbol e– for an electron. (Sub-topic – C4.1)
▶️Answer/Explanation
The balanced ionic half-equation for the formation of copper atoms from copper ions at the cathode is:
Cu2+ + 2e– → Cu
(d) Aluminium is a metal that is extracted by electrolysis. Fig. 11.2 shows the apparatus that is used. (Sub-topic – C4.1)

The word equation for the electrolysis of aluminium oxide is:
aluminium oxide → aluminium + oxygen
(i) State what is made at the cathode.
(ii) Oxide ions lose electrons to form oxygen molecules. The ionic half-equation for the reaction is:
2O2- – 4e– → O2
Electrons are lost during this process. State the name of this type of reaction.
▶️Answer/Explanation
(i) Aluminium is made at the cathode.
(ii) This type of reaction is called oxidation.
(e) Aluminium reacts with oxygen to make aluminium oxide, Al2O3: (Sub-topic – C3.3)
4Al + 3O2 → 2Al2O3
Calculate the maximum mass of aluminium oxide that can be made from 1.35 g of aluminium. Show your working.
▶️Answer/Explanation
First, calculate the number of moles of aluminium:
Moles of Al = mass / molar mass = 1.35 g / 27 g/mol = 0.05 mol
From the balanced equation, 4 moles of Al produce 2 moles of Al2O3. Therefore, 0.05 moles of Al will produce:
0.05 mol Al × (2 mol Al2O3 / 4 mol Al) = 0.025 mol Al2O3
Now, calculate the mass of Al2O3:
Mass of Al2O3 = moles × molar mass = 0.025 mol × 102 g/mol = 2.55 g
Therefore, the maximum mass of aluminium oxide that can be made from 1.35 g of aluminium is 2.55 g.
Question 12
α-particles, β-particles and γ-rays are all forms of ionising radiation.
(a) State one effect of ionising radiation on living things. (Sub-topic – P5.2.1)
▶️Answer/Explanation
Ionising radiation can cause damage to living cells, leading to mutations, cancer, or cell death. It can also affect DNA, potentially causing genetic mutations that may be passed on to future generations.
(b) The radioactive isotope uranium-238 decays into the isotope thorium-234 by emitting an α-particle. Use the correct nuclide notation to complete the decay equation for uranium-238. (Sub-topic – P5.2.2)

▶️Answer/Explanation
The decay equation for uranium-238 emitting an α-particle is:
\[ \frac{238}{92} U \rightarrow \frac{234}{90} Th + \frac{4}{2} He \]
Here, uranium-238 decays into thorium-234 by emitting an α-particle, which is a helium nucleus (\(\frac{4}{2} He\)).
(c) Gamma radiation is part of the electromagnetic spectrum. (Sub-topic – P3.3)
(i) State the speed of gamma radiation in a vacuum.
▶️Answer/Explanation
The speed of gamma radiation in a vacuum is \(3 \times 10^8 \, \text{m/s}\), which is the speed of light.
(ii) Draw lines to match each form of electromagnetic radiation to its use. One line has been drawn for you.

▶️Answer/Explanation
The correct matching is:
- Infrared – remote controls and intruder alarms
- Microwaves – satellite television and telephones
- Radio waves – radio and TV communications
- X-rays – medicine and security
(d) Visible light is also part of the electromagnetic spectrum. Fig. 12.1 shows an object emitting visible light and a thin converging lens. (Sub-topic – P3.2.3)

(i) Complete Fig. 12.1 to show how the rays of light from the object form an image.
▶️Answer/Explanation
To complete the diagram, draw two rays from the object:
- One ray parallel to the principal axis, which refracts through the lens and passes through the focal point on the other side.
- Another ray passing through the focal point on the object side, which refracts parallel to the principal axis after passing through the lens.
The point where these two rays intersect after refraction is the location of the real image formed by the lens.
(ii) The image formed is a real image. State one difference between a real image and a virtual image.
▶️Answer/Explanation
A real image is formed when light rays actually converge at a point, and it can be projected onto a screen. A virtual image, on the other hand, is formed when light rays appear to diverge from a point, and it cannot be projected onto a screen.
