Question-1 (a) :2019-may-Chemistry_paper_2__TZ1_SL
Topic:
Xylene is a derivative of benzene. One isomer is 1,4-dimethylbenzene.
Discuss: the number of ${ }^1 \mathrm{H}$ NMR signals for this isomer of xylene and the ratio in which they appear.
Number of signals:
Ratio:
▶️Answer/Explanation
Solution:
$
\begin{aligned}
&\text{In benzene ring}=1 \text { signal } \\
& -\mathrm{CH}_3=1 \text { signal } \\
\end{aligned}
$
$\rightarrow$ gives 2 different signals overall
There are 2 types of hydrogen atoms present in the given isomer of xylene, and thus there will be 2 signals in the ${ }^1 \mathrm{H}$ NMR spectrum.
The ratio of the signals depends on the number of hydrogen atoms associated with each type of carbon atom. In the given isomer, there are two types of carbon atoms: those that are directly attached to one methyl group, and those that are directly attached to two methyl groups. The former has two hydrogen atoms attached, while the latter has only one hydrogen atom attached.
Therefore, the two hydrogen atoms attached to the first type of carbon atom will give a singlet signal, while the hydrogen atoms attached to the second type of carbon atom will give a doublet signal.
The ratio of the singlet to doublet signals will be 6:4, or 3:2, because there are 6 hydrogen atoms attached to the first type of carbon atom and 4 hydrogen atoms attached to the second type of carbon atom.
Question-1(b) :2019-may-Chemistry_paper_2__TZ1_SL
Topic:
Draw: the structure of one other isomer of xylene which retains the benzene ring.
▶️Answer/Explanation
Solution:
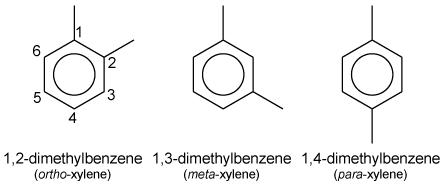
The three isomers of xylene are ortho-xylene (1,2-dimethylbenzene), meta-xylene (1,3-dimethylbenzene), and para-xylene (1,4-dimethylbenzene). They differ in the positions of the two methyl groups on the benzene ring.
Question-1[(c) (i)] :2019-may-Chemistry_paper_2__TZ1_SL
Topic:
Given: Bromine reacts with alkanes.
Discuss: the initiation step of the reaction and its conditions.
▶️Answer/Explanation
Solution:
The initiation step of the reaction between bromine and alkanes involves the homolytic cleavage (or homolysis) of the bromine molecule into two bromine radicals, each carrying one unpaired electron. This is typically achieved by providing energy to the system in the form of heat or light.
The equation for the initiation step can be represented as:
$\operatorname{Br}_ 2(\mathrm{~g}) \rightarrow 2 \mathrm{Br}\boldsymbol{\cdot}(\mathrm{g})$
The conditions for the initiation step depend on the specific alkane and reaction conditions being used. In general, the energy required for homolytic cleavage of the bromine molecule is provided by heat or light, and the reaction is typically carried out in the gas phase or in a non-polar solvent. The initiation step is usually the slowest step in the reaction, and its rate is dependent on the concentration of the reactants and the intensity of the energy source used to initiate the reaction.
Question-1[(c) (ii)] :2019-may-Chemistry_paper_2__TZ1_SL
Topic:
Given: 1,4-dimethylbenzene reacts as a substituted alkane.
Draw: the structures of the two products of the overall reaction when one molecule of bromine reacts with one molecule of 1,4-dimethylbenzene.
▶️Answer/Explanation
Solution:
alkane $+\mathrm{X}_2 \stackrel{\text { uv light }}{\longrightarrow} \text { mono-substituted haloalkane }+\mathrm{HX}$
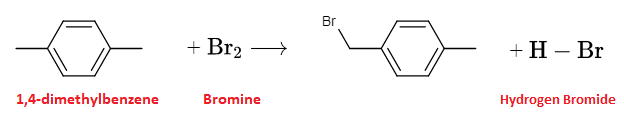
The reaction between 1,4-dimethylbenzene and $\mathrm{Br_2}$ under UV light would indeed lead to the formation of 1-bromo-4-methylbenzene and $\mathrm{H-Br}$ as a byproduct. Here are the balanced chemical equations for the reaction:
Initiation:
$\mathrm{Br_2}$ → 2$\mathrm{Br·}$
Propagation:
Step 1: $\mathrm{Br·}$ + 1,4-dimethylbenzene → 1-bromo-4-methylbenzyl radical + $\mathrm{HBr}$
Step 2: 1-bromo-4-methylbenzyl radical + $\mathrm{Br_2}$ → 1-bromo-4-methylbenzene + $\mathrm{Br·}$
Overall reaction:
1,4-dimethylbenzene + $\mathrm{Br_2}$ → 1-bromo-4-methylbenzene + $\mathrm{HBr}$
The substitution of one of the hydrogen atoms on the benzene ring with a bromine atom leads to the formation of 1-bromo-4-methylbenzene, which is a mono-substituted haloalkane.
Question-2(a) :2019-may-Chemistry_paper_2__TZ1_SL
Topic:
Given: Benzoic acid, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{COOH}$, is another derivative of benzene.
Identify: the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
▶️Answer/Explanation
Solution:
Infrared spectroscopy is a powerful analytical technique used to identify and characterize the functional groups present in a molecule. In IR spectroscopy, a beam of infrared radiation is passed through a sample, and the intensity of the transmitted radiation is measured as a function of the wavelength or wavenumber of the radiation.
The functional groups in a molecule absorb infrared radiation at specific frequencies that correspond to the stretching and bending vibrations of the bonds within the functional groups. These absorptions appear as peaks in the IR spectrum, which can be used to identify the types of functional groups present in the molecule.
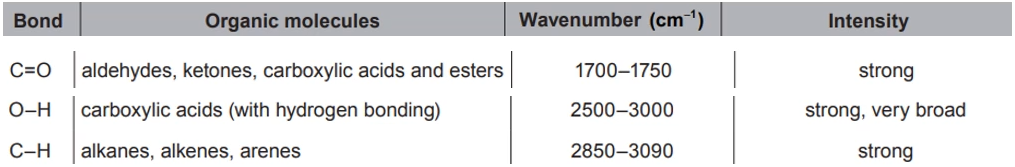
In the case of benzoic acid, one of the prominent peaks in the IR spectrum appears in the range of 2500-3000 $\mathrm{cm}^{-1}$, which corresponds to the stretching vibrations of the $\mathrm{C-H}$ bonds in the aromatic ring. This peak arises because the $\mathrm{C-H}$ bonds in the ring are relatively weak and can be excited to higher energy levels by the absorption of infrared radiation. The exact wavenumber of this peak depends on the specific structure of the molecule and the environment of the $\mathrm{C-H}$ bonds, but it falls within the range of 2500-3000 $\mathrm{cm}^{-1}$ for benzoic acid.
