Question-1(a) :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Given: Ethyne, $\mathrm{C}_2 \mathrm{H}_2$, reacts with oxygen in welding torches.
Write: an equation for the complete combustion of ethyne.
▶️Answer/Explanation
Solution:
The complete combustion of ethyne can be represented by the following equation:
$$\mathrm{C_2H_2 + 2.5O_2 \rightarrow 2CO_2 + H_2O}$$
In this reaction, ethyne reacts with oxygen gas to produce carbon dioxide and water. The stoichiometric coefficients in the balanced equation indicate that two moles of ethyne require 2.5 moles of oxygen to completely react.
Question-1[(b) (i) ] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Draw: the Lewis (electron dot) structure of ethyne.
▶️Answer/Explanation
Solution:
Ethyne is a hydrocarbon with the chemical formula $\mathrm{C_2H_2}$. It is also known as acetylene and is a colorless gas with a distinct odor. It is highly flammable and is commonly used as a fuel for welding and cutting torches.
Ethyne is an unsaturated hydrocarbon, meaning it contains one or more double or triple bonds between carbon atoms. In the case of ethyne, there is a triple bond between the two carbon atoms. This triple bond is composed of two pi bonds and one sigma bond.
Ethyne is produced naturally by certain bacteria, but it is also synthesized industrially from methane and other hydrocarbons through a process called cracking. It is an important chemical in the production of many organic compounds, including plastics, solvents, and synthetic rubber.
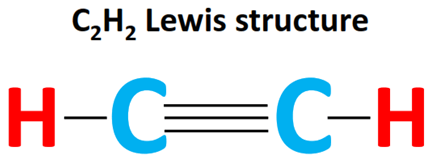
Question-1[(b) (ii)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Compare: giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, $\mathrm{C}_2 \mathrm{H}_6$.
▶️Answer/Explanation
Solution:
The bond length between the carbon atoms in ethyne is shorter than the bond length between the carbon atoms in ethane.
The bond length is the distance between the nuclei of the two atoms that are bonded together. In ethyne, the triple bond between the two carbon atoms consists of one sigma bond and two pi bonds. These bonds are shorter and stronger than the single covalent bond between the two carbon atoms in ethane. In addition, the triple bond in ethyne also results in a greater electron density between the two carbon atoms, which further contributes to a shorter bond length.
Therefore, the bond length between the carbon atoms in ethyne is shorter than that in ethane, which only has a single bond between the two carbon atoms.
Question-1[(b) (iii)] :2019-may-Chemistry_paper_2__TZ1_SL
Topic:
Discuss: the type of interaction that must be overcome when liquid ethyne vaporizes.
▶️Answer/Explanation
Solution:
When liquid ethyne vaporizes, the type of interaction that must be overcome is intermolecular forces.
Intermolecular forces are the attractive or repulsive forces that exist between molecules in a substance. In the liquid phase, the molecules of ethyne are held together by intermolecular forces, which keep them close together and in a fixed position. When ethyne vaporizes, these intermolecular forces are overcome and the molecules break away from one another, resulting in the formation of a gas.
In the case of ethyne, the dominant intermolecular force is London dispersion force. This force arises due to temporary fluctuations in electron density that create temporary dipoles, which attract neighboring molecules. The strength of London dispersion force depends on the size and shape of the molecule, as well as the number of electrons. In the case of ethyne, the molecules are small and have a linear shape, so the intermolecular forces are relatively weak, and the boiling point of ethyne is low (-84°C).
Question-1[(c) (i)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Ethyne reacts with steam.
$$
\mathrm{C}_2 \mathrm{H}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{C}_2 \mathrm{H}_4 \mathrm{O}(\mathrm{g})
$$
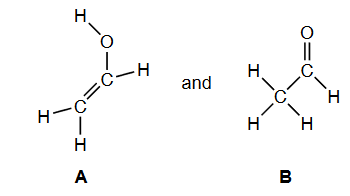
Product A contains a carbon-carbon double bond. State the type of reactions that compounds containing this bond are likely to undergo.
▶️Answer/Explanation
Solution:
Compounds containing a carbon-carbon double bond, such as Product A which contains a C=C double bond, are likely to undergo electrophilic addition reactions. In an electrophilic addition, an electrophile (an electron-deficient species) is attracted to the pi bond of the double bond, breaking it and forming two new single bonds. The electrophile then becomes bonded to one of the carbon atoms of the former double bond, while the other carbon atom becomes bonded to a nucleophile (an electron-rich species) or a proton. Electrophilic addition reactions are common in organic chemistry and are important in the synthesis of many organic compounds.
Question-1[(c) (ii)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Write: the name of product $\mathbf{B}$, applying IUPAC rules.
▶️Answer/Explanation
Solution:
The name of Product B, applying IUPAC rules, is “ethanal”.
The structure of Product B can be represented as follows:
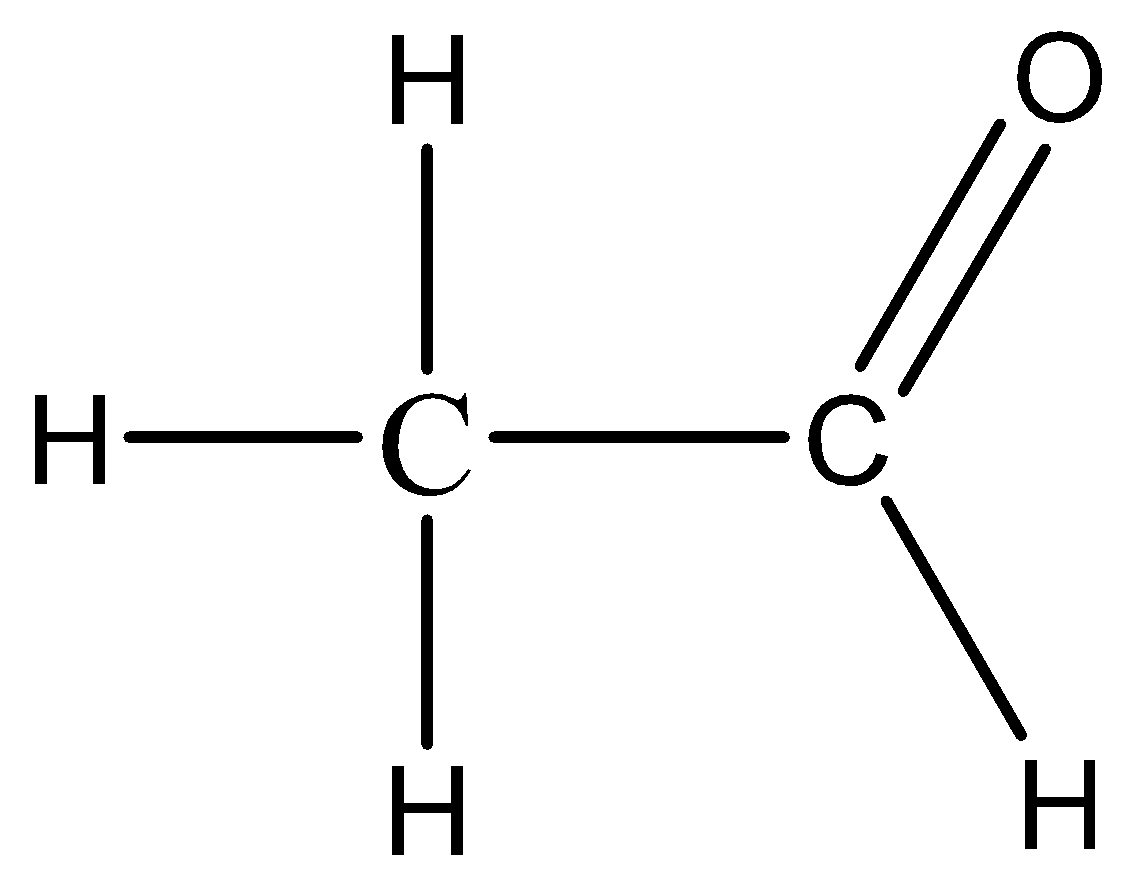
In the IUPAC nomenclature system, aldehydes are named by replacing the “-e” suffix of the corresponding parent alkane with the suffix “-al”. The parent alkane in this case is ethane, so the corresponding aldehyde is named “ethanal”.
Question-1[(c) (iii)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Calculate: the enthalpy change for the reaction, in kJ, to produce $\mathbf{A}$ using section 11 of the data booklet.
▶️Answer/Explanation
Solution:
We can use the bond enthalpies listed in Section 11 of the data booklet to estimate the enthalpy change for the reaction to produce Product A.
The bonds that are broken are: $1 \mathrm{C}-\mathrm{C}$ triple bond, $1 \mathrm{C}-\mathrm{H}$ bonds, and $2 \mathrm{O}-\mathrm{H}$ bond.
The bonds that are formed are: $3 \mathrm{C}-\mathrm{H}$ bonds , $1 \mathrm{C}-\mathrm{C}$ double bond, $1 \mathrm{C}-\mathrm{O}$ bond, and $1 \mathrm{H}-\mathrm{O}$ bonds.
The bond enthalpies we need are:
$\mathrm{C}-\mathrm{C}$ triple bond: $839 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{C}-\mathrm{H}$ bond: $414 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{O}-\mathrm{H}$ bond: $463 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{C}-\mathrm{C}$ double bond: $614 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{C}-\mathrm{O}$ bond: $358 \mathrm{~kJ} / \mathrm{mol}$
$\mathrm{H}-\mathrm{O}$ bond: $463 \mathrm{~kJ} / \mathrm{mol}$
The total energy required to break the bonds is:
$$
(1 \times 839 \mathrm{~kJ} / \mathrm{mol})+(2 \times 414 \mathrm{~kJ} / \mathrm{mol})+(2 \times 463 \mathrm{~kJ} / \mathrm{mol})=2593 \mathrm{~kJ} / \mathrm{mol}
$$
The total energy released by forming the new bonds is:
$$
(3 \times 414 \mathrm{~kJ} / \mathrm{mol})(1 \times 614 \mathrm{~kJ} / \mathrm{mol})+(1 \times 358 \mathrm{~kJ} / \mathrm{mol})+(1 \times 463 \mathrm{~kJ} / \mathrm{mol})=2677 \mathrm{~kJ} / \mathrm{mol}
$$
Therefore, the estimated enthalpy change for the reaction is:
$\Delta \mathrm{H}=$ (energy required to break bonds) – (energy released by forming bonds)
$$
\begin{aligned}
& =2593 \mathrm{~kJ} / \mathrm{mol}-2677 \mathrm{~kJ} / \mathrm{mol} \\
& =-84 \mathrm{~kJ} / \mathrm{mol}
\end{aligned}
$$
The enthalpy change for the reaction to produce Product $\mathrm{A}$ is estimated to be $-84 \mathrm{~kJ} / \mathrm{mol}$.
Question-1[(c) (iv)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Given: The enthalpy change for the reaction to produce $\mathbf{B}$ is $-213 \mathrm{~kJ}$.
Discuss: By giving a reason, which product is the most stable.
▶️Answer/Explanation
Solution:
The enthalpy change for the reaction to produce Product B is -213 kJ/mol, which means the reaction releases energy and is exothermic. In contrast, the enthalpy change for the reaction to produce Product A is -84 kJ/mol, which is also exothermic but releases less energy than the reaction to produce Product B.
Therefore, we can predict that Product B is more stable than Product A, since it releases more energy upon formation. The reason for this is that Product B, ethanal, has a stable carbonyl group (C=O bond) which is more thermodynamically favorable than a carbon-carbon double bond. The C=O bond is shorter and stronger than the C=C bond, and it has a higher bond energy, which makes it more stable. Therefore, the reaction to produce Product B, which forms a C=O bond, releases more energy than the reaction to produce Product A, which forms a C=C bond.
Question-1[(c) (v)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Given: The IR spectrum and low resolution ${ }^1 \mathrm{HNMR}$ spectrum of the actual product formed are shown.
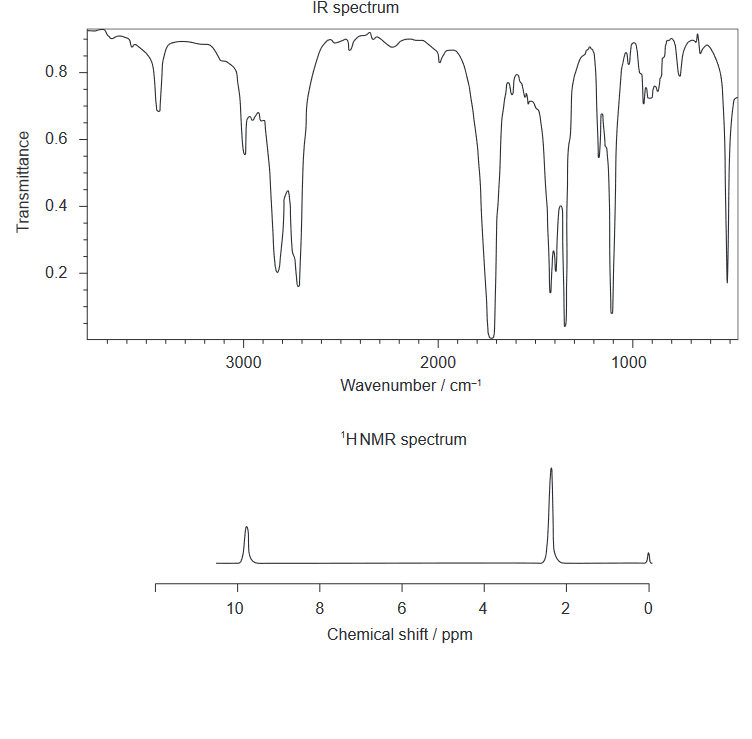
Discuss: whether the product is $\mathbf{A}$ or $\mathbf{B}$, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:…………………….
One piece of evidence from IR:.………………….
One piece of evidence from ${ }^1 \mathrm{HNMR}$ :………………………
▶️Answer/Explanation
Solution:
To determine whether the product formed is A or B, we can use the evidence from the IR spectrum and ${}^1\mathrm{HNMR}$ spectrum and compare them to the data in Sections 26 and 27 of the data booklet.
From the IR spectrum, we can see that there is a strong peak at around 1700 $\mathrm{cm^{-1}}$, which corresponds to a C=O stretching vibration. This indicates that the product contains a carbonyl group, which is a characteristic feature of Product B (ethanal). Product A does not contain a carbonyl group, so it cannot be the product formed.
From the ${}^1\mathrm{HNMR}$ spectrum, we can see that there are three distinct peaks at around 2 ppm, 2.5 ppm, and 3 ppm, which correspond to the chemical shifts of the protons in the product. Using the chemical shift values in Section 27 of the data booklet, we can identify the types of protons in the product.
The peak at 2 ppm corresponds to the protons on the methyl group (-CH3) of the product, which is consistent with both Products A and B.
The peak at 2.5 ppm corresponds to the protons on the methylene group (-CH2-) of the product, which is also consistent with both Products A and B.
The peak at 3 ppm corresponds to the proton on the carbonyl group (-C=O) of the product, which is a characteristic feature of Product B (ethanal). Product A does not contain a carbonyl group, so it cannot have a peak in this region.
Therefore, based on the evidence from the IR and ${}^1\mathrm{HNMR}$ spectra and the data in Sections 26 and 27 of the data booklet, we can conclude that the product formed is most likely Product B (ethanal).
Identity of product: ethanal
One piece of evidence from IR: Strong peak at around 1700 $\mathrm{cm^{-1}}$ corresponding to a C=O stretching vibration
One piece of evidence from ${}^1\mathrm{HNMR}$: Peak at 3 ppm corresponding to the proton on the carbonyl group (-C=O) of the product.
Question-1[(d) (i)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Given: Product $\mathrm{B}, \mathrm{CH}_3 \mathrm{CHO}$, can also be synthesized from ethanol.
Discuss: the reagents and conditions required to ensure a good yield of product $\mathbf{B}$.
Reagents:
Conditions:
▶️Answer/Explanation
Solution:
Reagents:
1. Concentrated sulfuric acid ($\mathrm{H}_2\mathrm{SO}_4$)
2. Acidic aqueous potassium dichromate ($\mathrm{K}_2\mathrm{Cr}_2\mathrm{O}_7$)
Conditions:
1. Reflux
2. Heat
In the first step of the reaction, ethanol is dehydrated to form ethene. This is done by mixing ethanol with concentrated sulfuric acid and heating the mixture under reflux conditions. The sulfuric acid acts as a catalyst, promoting the dehydration of ethanol to ethene. The reaction can be represented as:
$\mathrm{CH}_3\mathrm{CH}_2\mathrm{OH} \rightarrow \mathrm{CH}_2= \mathrm{CH}_2 + \mathrm{H}_2\mathrm{O}$
In the second step of the reaction, the ethene produced in the first step is oxidized to ethanal using acidic aqueous potassium dichromate (VI). The reaction can be represented as:
$\mathrm{CH}_2= \mathrm{CH}_2 + \mathrm{H}_2\mathrm{O} + \mathrm{[O]} \rightarrow \mathrm{CH}_3\mathrm{CHO}$
The oxidation is carried out under heat and reflux conditions. The acidic aqueous potassium dichromate (VI) acts as an oxidizing agent, oxidizing the ethene to form ethanal. The use of reflux ensures that the reaction mixture is maintained at a constant temperature, which helps to improve the yield of the product.
Question-1[(d) (ii)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Discuss: the average oxidation state of carbon in product $\mathbf{B}$.
▶️Answer/Explanation
Solution:
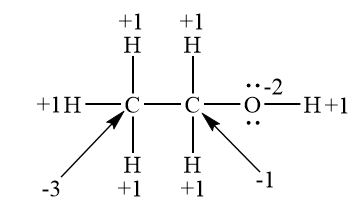
$\text { Oxidation state: -1 }$
Question-1[(d) (iii)] :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Discuss: why product$\mathbf{B}$ is water soluble.
▶️Answer/Explanation
Solution:
Product B, $\mathrm{CH_3CHO}$ or acetaldehyde, is water-soluble due to its polar nature. Acetaldehyde contains a polar carbonyl group, $\mathrm{C=O}$, which imparts a significant dipole moment to the molecule. The partial positive charge on the carbonyl carbon and the partial negative charge on the oxygen atom make the molecule polar.
When acetaldehyde is placed in water, the polar water molecules surround the polar acetaldehyde molecules and interact with them through dipole-dipole interactions and hydrogen bonding. The oxygen atom in water is partially negative and is attracted to the partial positive charge on the carbonyl carbon of acetaldehyde. Similarly, the hydrogen atoms in water are partially positive and are attracted to the partial negative charge on the oxygen atom of acetaldehyde. These interactions allow acetaldehyde to dissolve in water and make it water-soluble.
Question-2 (a) :2019-may-Chemistry_paper_2__TZ2_SL
Topic:
Given: The thermal decomposition of dinitrogen monoxide occurs according to the equation:
$$
2 \mathrm{~N}_2 \mathrm{O}(\mathrm{g}) \rightarrow 2 \mathrm{~N}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})
$$
The reaction can be followed by measuring the change in total pressure, at constant temperature, with time.
The $x$-axis and $y$-axis are shown with arbitrary units.
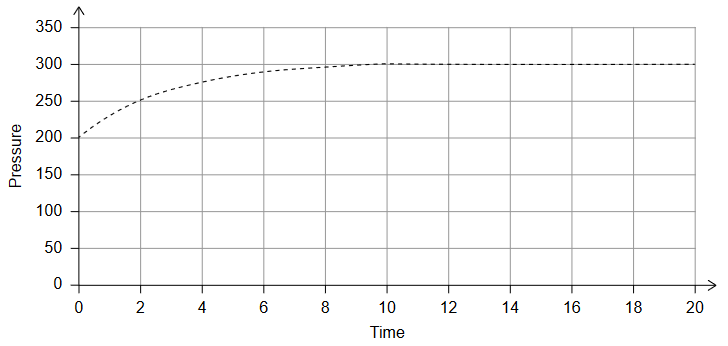
Explain: why, as the reaction proceeds, the pressure increases by the amount shown.
▶️Answer/Explanation
Solution:
In the given graph, the pressure increases with time as the dinitrogen monoxide decomposes to nitrogen and oxygen. This increase in pressure can be explained by the ideal gas law, which states that the pressure of a gas is directly proportional to the number of gas molecules present, as well as their temperature and volume.
In the beginning, the total pressure is due to the presence of only dinitrogen monoxide gas molecules. As the decomposition reaction proceeds, the number of gas molecules increases because two molecules of dinitrogen monoxide produce two molecules of nitrogen gas and one molecule of oxygen gas. Therefore, the total number of gas molecules increases, leading to an increase in pressure.
Additionally, the volume of the reaction vessel remains constant, and the temperature is held constant. As a result, any change in pressure can only be due to a change in the number of gas molecules. Therefore, the increase in pressure observed in the graph is due to the increase in the number of gas molecules as the reaction proceeds.
