Q.1(a).2022-May-Physics_paper_1__TZ2_HL
Topic: Forces
Given: Airboats are used for transport across a river. To move the boat forward, air is propelled from the back of the boat by a fan blade.
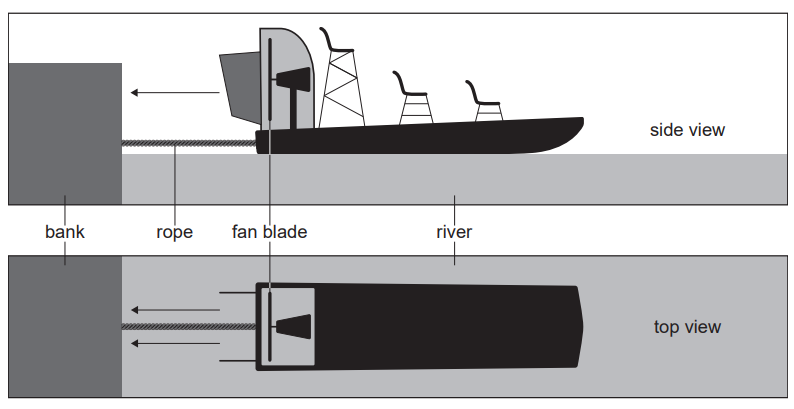
An airboat has a fan blade of radius $1.8 \mathrm{~m}$. This fan can propel air with a maximum speed relative to the boat of $20 \mathrm{~ms}^{-1}$. The density of air is $1.2 \mathrm{~kg} \mathrm{~m}^{-3}$.
Outline: why a force acts on the airboat due to the fan blade.
Answer/Explanation
Solution:
Q.1[(b) (i)] .2022-May-Physics_paper_1__TZ2_HL
Topic: Forces
Given: In a test the airboat is tied to the river bank with a rope normal to the bank. The fan propels the air at its maximum speed. There is no wind.
Calculate: Show that a mass of about $240 \mathrm{~kg}$ of air moves through the fan every second.
Answer/Explanation
Solution:
We can calculate the mass of air moving through the fan every second using the formula:
$$\text{mass} = \text{density} \times \text{volume}$$
The volume of air moving through the fan per second is equal to the volume of air swept out by the fan blades per second. The volume of air swept out by the fan blades per second can be calculated using the formula for the volume of a cylinder:
$$\text{volume} = \pi r^2 v$$
where $r$ is the radius of the fan blade and $v$ is the speed of the air relative to the boat.
Substituting the given values, we get:
$$\text{volume} = \pi (1.8 \mathrm{~m})^2 (20 \mathrm{~ms}^{-1}) \approx 203 \mathrm{~m}^3 \mathrm{s}^{-1}$$
Substituting the given density of air, we get:
$$\colorbox{yellow}{$\text{mass} \approx (1.2 \mathrm{~kg} \mathrm{~m}^{-3}) \times (203 \mathrm{~m}^3 \mathrm{s}^{-1}) \approx 244 \mathrm{kgs}^{-1}$}$$
Q.1[(b) (ii)].2022-May-Physics_paper_1__TZ2_HL
Topic: Forces
Calculate: Show that the tension in the rope is about $5 \mathrm{kN}$.
Answer/Explanation
Solution:
When the fan propels air at its maximum speed, it exerts a force on the air, and the air exerts an equal and opposite force on the fan blade. By Newton’s third law, the airboat experiences an equal and opposite force, which propels it forward.
However, in this case, the airboat is tied to the river bank with a rope normal to the bank. The tension in the rope balances the force on the boat due to the air. Therefore, we can find the tension in the rope by calculating the force on the boat due to the air.
The force on the boat due to the air can be calculated using the formula:
$$\text{force} = \text{mass} \times \text{acceleration}$$
where mass is the mass of air moving through the fan every second, and acceleration is the acceleration of the air relative to the boat.
From part (b)(i), we know that the mass of air moving through the fan every second is about $240 \mathrm{~kg}$. The acceleration of the air relative to the boat is the maximum speed of the air relative to the boat, which is $20 \mathrm{~ms}^{-1}$.
Therefore, the force on the boat due to the air is:
$$\colorbox{yellow}{$\text{force} = (244 \mathrm{~kg}) \times (20 \mathrm{~ms}^{-1}) \approx 4900 \mathrm{~N}$}$$
Q.1[(c) (i)].2022-May-Physics_paper_1__TZ2_HL
Topic: Motion
Given: The rope is untied and the airboat moves away from the bank. The variation with time $t$ of the speed $v$ of the airboat is shown for the motion.
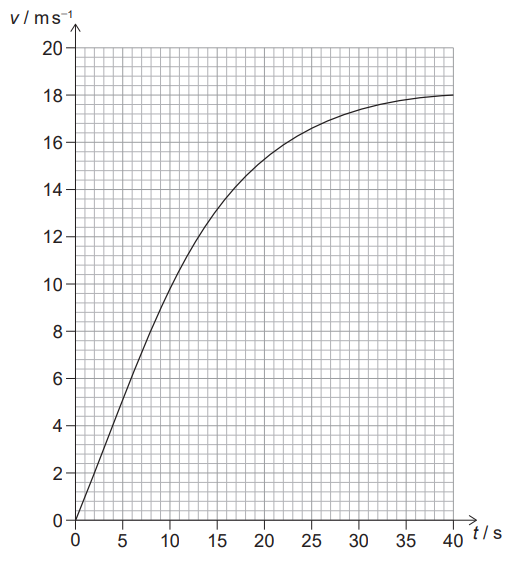
Explain: why the airboat has a maximum speed under these conditions.
Answer/Explanation
Solution:
The maximum speed of the airboat occurs when the force on the airboat due to the fan blade is balanced by the resistive forces acting on the boat, such as frictional forces between the boat and the water, and drag forces due to the air resistance.
Initially, when the rope is untied, there is no resistive force acting on the boat, and the fan blade accelerates air backward, propelling the boat forward. As the boat gains speed, the resistive forces increase, and the acceleration of the boat decreases. Eventually, the resistive forces balance the force on the boat due to the fan blade, and the boat reaches its maximum speed. At this speed, the force on the boat due to the fan blade is balanced by the resistive forces, so the net force on the boat is zero, and it moves at a constant speed. Any further increase in the force on the boat due to the fan blade would result in an increase in the net force on the boat, and hence an increase in the acceleration of the boat, rather than an increase in the speed.
Therefore, the airboat has a maximum speed under these conditions because the force on the boat due to the fan blade is balanced by the resistive forces acting on the boat, resulting in zero net force and constant speed.
Q.1[(c) (ii)].2022-May-Physics_paper_1__TZ2_HL
Topic: Motion
Estimate: the distance the airboat travels to reach its maximum speed.
Answer/Explanation
Solution:
Area under the graph is distance covered:
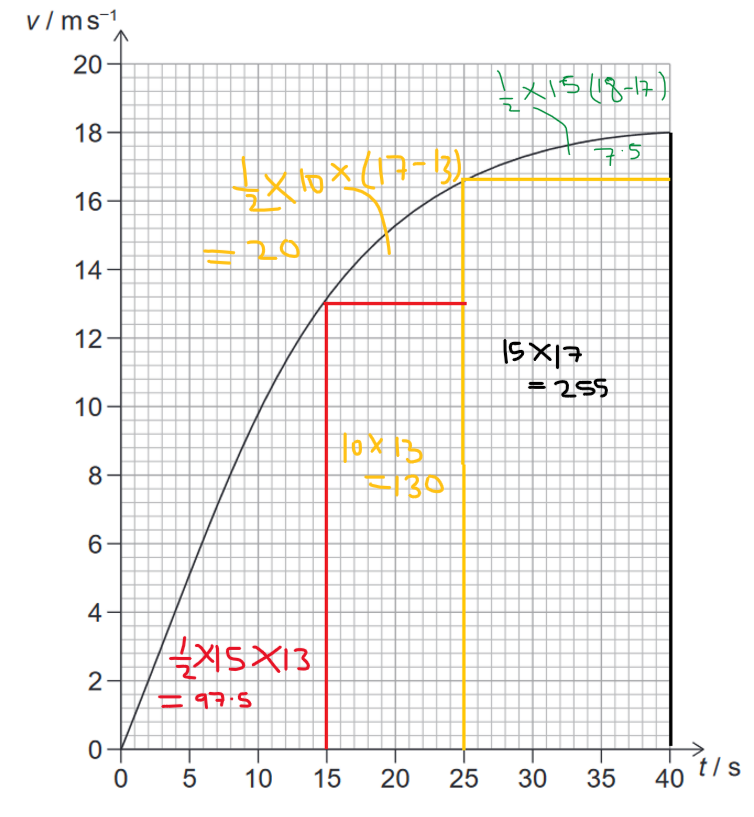
$\colorbox{yellow}{$\text{Area / distance covered}=97.5+130+20+255+7.5=510 \mathrm{~m}$}$
Q.1[(c) (iii)].2022-May-Physics_paper_1__TZ2_HL
Topic: Forces
Calculate: Deduce the mass of the airboat.
Answer/Explanation
Solution:
We have to find initial acceleration of boat. So we will calculate of acceleration as gradient at $t=0\ to\ t=1 \Rightarrow a= 1\mathrm{~m} \mathrm{~s}^{-2}$
using $F=ma$
$m=\frac{4900}{1}$
Q.2(a).2022-May-Physics_paper_1__TZ2_HL
Topic: Modelling a gas
Given: A fixed mass of an ideal gas is contained in a cylinder closed with a frictionless piston. The volume of the gas is $2.5 \times 10^{-3} \mathrm{~m}^3$ when the temperature of the gas is $37^{\circ} \mathrm{C}$ and the pressure of the gas is $4.0 \times 10^5 \mathrm{~Pa}$.
Calculate: the number of gas particles in the cylinder.
Answer/Explanation
Solution:
We can use the ideal gas law to relate the number of moles of gas, the temperature, the pressure, and the volume of the gas:
$$PV = nRT$$
where $P$ is the pressure of the gas, $V$ is the volume of the gas, $n$ is the number of moles of gas, $R$ is the gas constant, and $T$ is the temperature of the gas in kelvin.
We are given the volume $V = 2.5 \times 10^{-3} \mathrm{~m}^3$, the temperature $T = 37^{\circ} \mathrm{C} = 310 \mathrm{~K}$ (converted to kelvin), and the pressure $P = 4.0 \times 10^5 \mathrm{~Pa}$. The gas constant $R$ is a known value: $R = 8.31 \mathrm{~J} \mathrm{mol}^{-1} \mathrm{K}^{-1}$.
Substituting these values, we get:
$$n = \frac{PV}{RT} = \frac{(4.0 \times 10^5 \mathrm{~Pa})(2.5 \times 10^{-3} \mathrm{~m}^3)}{(8.31 \mathrm{~J} \mathrm{mol}^{-1} \mathrm{K}^{-1})(310 \mathrm{~K})} \approx 0.388 \mathrm{~mol}$$
Therefore, there are about 0.033 moles of gas in the cylinder.
To calculate the number of gas particles, we can use Avogadro’s number $N_A = 6.022 \times 10^{23} \mathrm{~mol}^{-1}$:
