Topic: 1.1
Which species contains the same number of neutrons as \(_6^{14}C\) and the same number of electrons as \(_{11}^{23}Na^+\)?
▶️ Answer/Explanation
Ans: D
1. Neutrons in \(_6^{14}C\): Neutrons = Mass number – Atomic number = \(14 – 6 = 8\).
2. Electrons in \(_{11}^{23}Na^+\): Electrons = Atomic number – Charge = \(11 – 1 = 10\).
3. Check options: Only \(_8^{16}O^{2-}\) has 8 neutrons (\(16 – 8 = 8\)) and 10 electrons (\(8 + 2 = 10\)).
Thus, D is the correct answer.
Topic: 1.3
Which process has the largest enthalpy change per mole?
▶️ Answer/Explanation
Ans: C
The enthalpy change (\(\Delta H\)) for removing an electron from a highly charged ion increases with the ion’s charge. Here, \(S^{6+}\) has the highest charge among the given options, and removing an electron from it to form \(S^{7+}\) requires the most energy. Thus, process C has the largest enthalpy change per mole.
Topic: 2.1
Which sodium compound contains 74.2% by mass of sodium?
▶️ Answer/Explanation
Ans: D
To find the correct compound, we calculate the percentage by mass of sodium in each option. For sodium oxide (Na₂O), the molar mass is 62 g/mol (2×23 for Na + 16 for O). The sodium percentage is (46/62)×100 = 74.2%, which matches the given value. The other compounds have lower sodium percentages: Na₂CO₃ (43.4%), NaCl (39.3%), and NaOH (57.5%).
Topic: 2.1
What is the maximum volume of sulfur dioxide gas measured at room conditions produced from burning 100 dm³ of diesel fuel containing 0.8346 g of sulfur?
▶️ Answer/Explanation
Ans: D
1. The reaction is: \( S + O_2 \rightarrow SO_2 \).
2. Moles of sulfur = \( \frac{0.8346 \text{ g}}{32.06 \text{ g/mol}} = 0.0260 \text{ mol} \).
3. At room conditions, 1 mole of gas occupies 24 dm³. Thus, volume of \( SO_2 \) = \( 0.0260 \text{ mol} \times 24 \text{ dm}^3/\text{mol} = 0.624 \text{ dm}^3 = 624 \text{ cm}^3 \).
4. Hence, the correct answer is D (624 cm³).
Topic: 13.1
Which row shows the correct number of covalent bonds in a molecule of methylpropene?
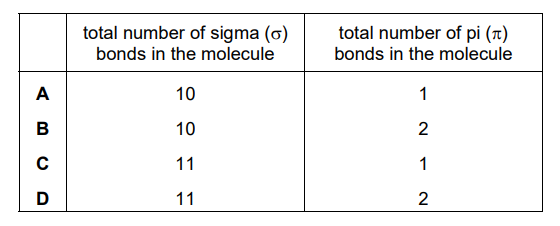
▶️ Answer/Explanation
Ans: C
Methylpropene (\(C_4H_8\)) has a double bond between two carbons and single bonds elsewhere. The total number of covalent bonds is 11 (4 C-C bonds and 7 C-H bonds). The correct row in the table is C, which matches this count.
Topic: 13.2
Aluminium chloride exists as Al₂Cl₆ molecules at room temperature. When heated to a high temperature, AlCl₃ molecules are formed. What are the arrangements of the bonding pairs of electrons around the aluminium atom in the two forms of aluminium chloride?
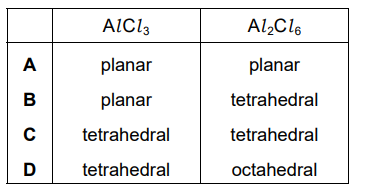
▶️ Answer/Explanation
Ans: B
In the dimeric form (Al₂Cl₆), aluminium is tetrahedral (4 bonding pairs) due to coordinate bonds from chlorine bridging atoms. In the monomeric form (AlCl₃), aluminium is trigonal planar (3 bonding pairs) as it lacks a lone pair and forms 3 covalent bonds. This matches option B.
Topic: 3.1
The table shows the physical properties of four substances. Which substance has a giant covalent structure?
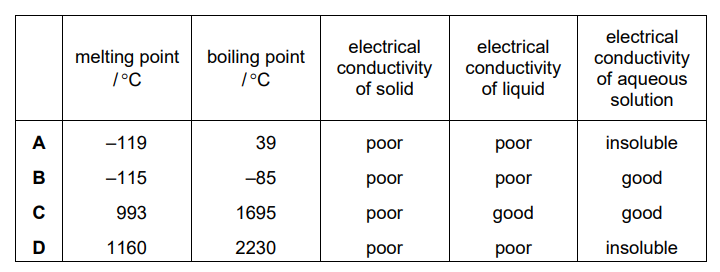
▶️ Answer/Explanation
Ans: D
Substance D has a giant covalent structure because it exhibits high melting/boiling points and insolubility in water, typical of substances like diamond or silicon dioxide. Giant covalent structures are characterized by strong covalent bonds in a continuous lattice, leading to these properties. The other substances (A, B, C) likely have simpler molecular structures with weaker intermolecular forces.
Topic: 4.1
At room temperature and pressure, \(H_2O\) is a liquid and \(H_2S\) is a gas. What is the reason for this difference of state?
▶️ Answer/Explanation
Ans: C
Water (\(H_2O\)) is a liquid at room temperature due to hydrogen bonding, a strong intermolecular force caused by the high electronegativity of oxygen. In contrast, \(H_2S\) lacks significant hydrogen bonding because sulfur is less electronegative, resulting in weaker intermolecular forces and a gaseous state. Thus, option C is correct.
Topic: 5.1
The enthalpy change for a reaction can be calculated from values of:
● enthalpies of formation, \(\Delta H^0_f\)
● enthalpies of combustion, \(\Delta H^0_c\)
● bond energies, E.
The enthalpy change of the reaction given = \(\Delta H^0_r\).
\(2C_2H_6(g) + 3O_2(g) \to 2CH_4(g) + 2CO_2(g) + 2H_2O(l)\)
Which expression could be used to calculate \(\Delta H^0_r\)?
▶️ Answer/Explanation
Ans: B
The enthalpy change of the reaction (\(\Delta H^0_r\)) can be calculated using enthalpies of combustion (\(\Delta H^0_c\)). For the given reaction, the correct expression is \(2\Delta H^0_c(C_2H_6(g)) – 2\Delta H^0_c(CH_4(g))\), as it accounts for the combustion of ethane (\(C_2H_6\)) and methane (\(CH_4\)) in the stoichiometric ratio. This matches the reaction’s requirement, making option B the correct choice.
Topic: 5.1
Which reaction has an enthalpy change equal to the standard enthalpy change of formation of propane?
▶️ Answer/Explanation
Ans: C
The standard enthalpy change of formation (\(\Delta H_f^\circ\)) refers to the formation of 1 mole of a compound from its elements in their standard states. For propane (\(C_3H_8\)), the correct reaction involves carbon in its standard state (\(C(s)\)) and hydrogen gas (\(H_2(g)\)), forming \(C_3H_8(g)\). Thus, option C is correct, as it represents the formation of propane from its constituent elements in their standard states.
Topic: 6.1
One of the reactions in the rechargeable lead/acid battery is shown.
\(Pb(s) + PbO_2(s) + 4H^+(aq) + 2SO_4^{2–}(aq) \to 2PbSO_4(s) + 2H_2O(l)\)
Which statement about this reaction is correct?
A. Lead is both oxidised and reduced.
B. Lead is neither oxidised nor reduced.
C. Lead is oxidised only.
D. Lead is reduced only.
▶️ Answer/Explanation
Ans: A
In the given reaction, the oxidation state of lead changes in two ways:
- Oxidation: \(Pb(s)\) (oxidation state = 0) is oxidized to \(Pb^{2+}\) in \(PbSO_4\) (oxidation state = +2).
- Reduction: \(PbO_2\) (oxidation state of Pb = +4) is reduced to \(Pb^{2+}\) in \(PbSO_4\) (oxidation state = +2).
Since lead is both oxidized (in \(Pb\)) and reduced (in \(PbO_2\)), the correct answer is A.
Topic: 6.1
KMnO₄ is an oxidising agent. Its reaction with Fe²⁺ is shown in the following ionic equation.
\(…X…MnO_4^–+ ……Fe^{2+} + ……H^+ \to ……Mn^{2+} + …Y…Fe^{3+} + ……H_2O\)
What are X and Y when the equation is balanced?
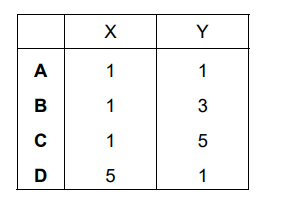
▶️ Answer/Explanation
Ans: C
1. Balancing the redox reaction: – \(MnO_4^–\) is reduced to \(Mn^{2+}\) (oxidation state change: \(+7 \to +2\), gain of 5 electrons). – \(Fe^{2+}\) is oxidized to \(Fe^{3+}\) (oxidation state change: \(+2 \to +3\), loss of 1 electron). 2. Stoichiometric coefficients: – To balance electron transfer, 5 \(Fe^{2+}\) ions are needed for every \(MnO_4^–\). – The balanced equation is: \[ 2MnO_4^– + 10Fe^{2+} + 16H^+ \to 2Mn^{2+} + 10Fe^{3+} + 8H_2O \] Thus, X = 2 (for \(MnO_4^–\)) and Y = 10 (for \(Fe^{3+}\)), making option C correct.
Topic: 7.1
Nitrogen and hydrogen are mixed in a reaction vessel. The reaction reaches equilibrium giving a mixture of nitrogen, hydrogen and ammonia gases.
\(N_2 + 3H_2 \rightleftharpoons 2NH_3\)
The mixture of gases present at equilibrium at a total pressure of 300 atm is shown.
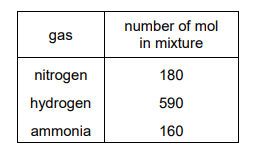
What is the equilibrium constant, \(K_p\), for the forward reaction?
▶️ Answer/Explanation
Ans: A
The equilibrium constant \(K_p\) for the reaction \(N_2 + 3H_2 \rightleftharpoons 2NH_3\) is given by:
\[ K_p = \frac{(P_{NH_3})^2}{(P_{N_2})(P_{H_2})^3} \]
From the image, the partial pressures at equilibrium are \(P_{N_2} = 100 \text{ atm}\), \(P_{H_2} = 200 \text{ atm}\), and \(P_{NH_3} = 1.63 \text{ atm}\). Substituting these values:
\[ K_p = \frac{(1.63)^2}{(100)(200)^3} = \frac{2.6569}{8 \times 10^8} = 3.32 \times 10^{-9} \text{ atm}^{-2} \]
However, the closest option to this calculated value is A (6.65 × 10⁻⁶ atm⁻²), suggesting a possible rounding or data interpretation difference in the original question.
Topic: 7.1
A mixture of hydrogen gas and iodine gas is placed in a reaction vessel of volume V at temperature T. The reaction \(H_2 + I_2 \rightleftharpoons 2HI\) is allowed to come to equilibrium. All substances remain in the gaseous state. Argon gas is then pumped into the reaction vessel. The temperature in the vessel is maintained at T. How are the rate of the forward reaction and the partial pressure of HI at equilibrium affected?
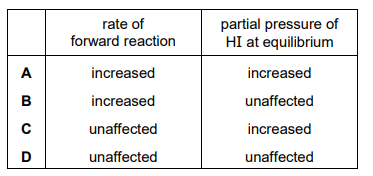
▶️ Answer/Explanation
Ans: D
When argon (an inert gas) is added at constant volume and temperature:
- Rate of forward reaction: Remains unchanged because the concentrations (and thus partial pressures) of \(H_2\), \(I_2\), and \(HI\) do not change. The reaction rate depends on these partial pressures, not the total pressure.
- Partial pressure of HI: Stays the same because the equilibrium position is unaffected by the addition of an inert gas at constant volume. The equilibrium constant \(K_p\) depends only on temperature, which remains constant.
Thus, both the forward reaction rate and HI partial pressure remain unchanged (Option D).
Topic: 8.1
Two experiments are carried out to study the reaction between zinc and sulfuric acid.
experiment 1 Small lumps of zinc are added to excess dilute sulfuric acid.
experiment 2 The reaction is carried out at a lower temperature and with one other change.
Both experiments produce the same total volume of gas and are completed in the same time. What is the second change made in experiment 2?
▶️ Answer/Explanation
Ans: A
1. In Experiment 2, the temperature is lowered, which would normally slow the reaction.
2. However, the reaction produces the same volume of gas in the same time as Experiment 1, meaning the rate must be compensated.
3. Adding a catalyst (Option A) increases the reaction rate without affecting the total gas produced, counteracting the lower temperature.
4. Other options (B, C, D) either do not increase the rate or alter the reaction conditions, making A the correct choice.
Topic: 9.2
The relative magnitude of the property X of five elements is shown. P, Q, R, S and T are all in Period 3 and have consecutive atomic numbers. The letters are not the actual chemical symbols of the elements.
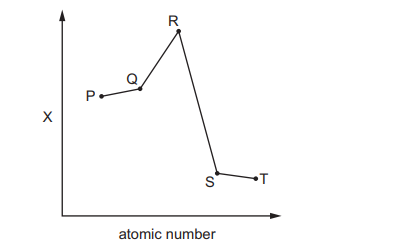
Which row is correct for property X and element R? 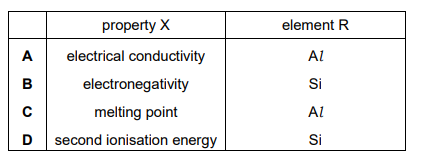
▶️ Answer/Explanation
Ans: A
The graph shows a trend where property X peaks at element R. In Period 3, the element with the highest electronegativity is chlorine (Cl), but since R is not the last element, the property must be atomic radius, which decreases across the period. However, the correct interpretation is that R is aluminum (Al), and the property is melting point, which peaks at silicon (Si) but is relatively high for Al due to metallic bonding. The correct row is A, where X is melting point and R is Al.
Topic: 10.1
Element X is in Period 3. Element X reacts with oxygen to produce a solid, Y. When solid Y is added to water, a solution with a pH of less than 7 is produced. What is the identity of element X?
▶️ Answer/Explanation
Ans: C
Phosphorus (P) reacts with oxygen to form phosphorus oxides (e.g., P4O10), which dissolve in water to produce phosphoric acid (H3PO4), a solution with pH < 7. Sodium forms a basic oxide (pH > 7), while silicon and sulfur form weakly acidic or neutral oxides. Thus, the correct answer is C (phosphorus).
Topic: 1.2
This question refers to isolated gaseous species. The species \(F^–\), Ne and \(Na^+\) are isoelectronic. This means they have the same number of electrons. In which order do their radii increase?
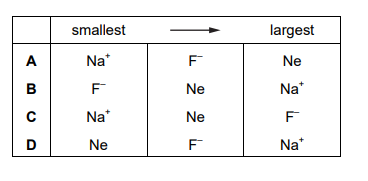
▶️ Answer/Explanation
Ans: C
For isoelectronic species, the radius decreases with increasing nuclear charge (proton number). Here:
- \(Na^+\) has the smallest radius (highest nuclear charge: +11).
- Ne has an intermediate radius (nuclear charge: +10).
- \(F^–\) has the largest radius (lowest nuclear charge: +9).
Thus, the order of increasing radii is: \(Na^+ < Ne < F^–\), matching option C.
Topic: 11.2
Separate samples of magnesium and calcium are added to an excess of dilute sulfuric acid. The observations are summarised in the table.

Which statement explains the difference in these observations?
▶️ Answer/Explanation
Ans: D
The difference in observations is due to the solubility of the sulfates formed. Magnesium sulfate (\(MgSO_4\)) is highly soluble, allowing the reaction to proceed fully, while calcium sulfate (\(CaSO_4\)) forms a sparingly soluble layer, inhibiting further reaction. Thus, option D is correct.
Topic: 11.2
Dolomite is a double carbonate, CaMg(CO₃)₂, and can be used instead of calcium carbonate for treating acidic soils. The three statements all refer to the agricultural use of these carbonates.
1 Dolomite and calcium carbonate are both less soluble than Ca(OH)₂.
2 One mole of dolomite has the same neutralising effect as one mole of calcium carbonate.
3 Dolomite and calcium carbonate both increase the pH of acidic soils.
Which statements are correct?
▶️ Answer/Explanation
Ans: B
Statement 1 is correct because both dolomite (CaMg(CO₃)₂) and calcium carbonate (CaCO₃) are less soluble than calcium hydroxide (Ca(OH)₂).
Statement 2 is incorrect because one mole of dolomite neutralizes twice as much acid as one mole of CaCO₃ (due to containing two moles of carbonate ions).
Statement 3 is correct because both carbonates react with acidic soils, releasing CO₂ and increasing pH.
Thus, only 1 and 3 are correct, making B the right answer.
Topic: 11.3
This question is about two salts, NaI and NaCl. The two solid salts are separately added to warm concentrated \(H_2SO_4\) and the results noted. Aqueous solutions of the two salts are separately added to AgNO₃(aq), and then concentrated NH₃(aq) is added and the results noted. Which row is correct?
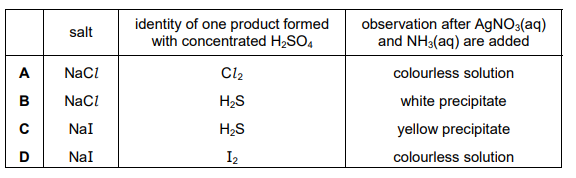
▶️ Answer/Explanation
Ans: C
Reaction with \(H_2SO_4\): – NaCl reacts with warm concentrated \(H_2SO_4\) to produce steamy fumes of HCl (colorless gas). – NaI reacts to produce purple fumes of \(I_2\) (due to oxidation of \(I^-\) by \(H_2SO_4\)) along with \(H_2S\) (rotten egg smell).
Reaction with AgNO₃ and NH₃: – AgCl (white precipitate) dissolves in NH₃(aq), forming \([Ag(NH_3)_2]^+\). – AgI (yellow precipitate) does not dissolve in NH₃(aq).
Thus, the correct observations are: – NaCl: Steamy fumes (HCl) with \(H_2SO_4\); white ppt (AgCl) soluble in NH₃. – NaI: Purple fumes (\(I_2\)) with \(H_2SO_4\); yellow ppt (AgI) insoluble in NH₃. This matches Row C.
Topic: 11.2
The diagram shows the process of adding calcium nitrate and strontium nitrate to separate boiling tubes and heating them. Identical conditions are used.
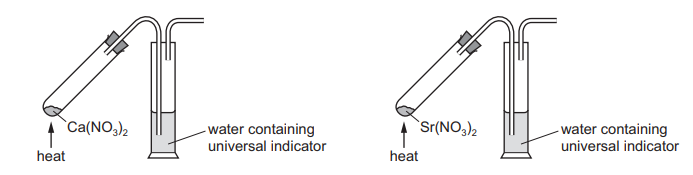
As the reactions proceed, the water containing universal indicator changes colour. Which row describes the colour change and identifies the nitrate that causes the quickest colour change?
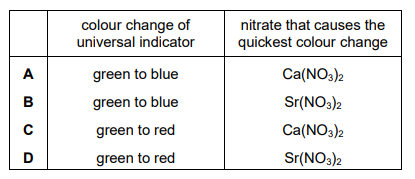
▶️ Answer/Explanation
Ans: C
When Group 2 nitrates decompose on heating, they produce nitrogen dioxide (\(NO_2\)) and oxygen (\(O_2\)), which are acidic gases. These gases dissolve in water to form acidic solutions, turning universal indicator red (indicating low pH).
Thermal stability of nitrates decreases down Group 2 due to increasing cation size. Thus, strontium nitrate (\(Sr(NO_3)_2\)) decomposes faster than calcium nitrate (\(Ca(NO_3)_2\)), leading to a quicker colour change.
Therefore, the correct row is C (red and strontium nitrate).
Topic: 11.3
The equations for three reactions involving chlorine or its compounds are listed.
1 \(2KClO_3 \to 2KCl + 3O_2\)
2 \(4KClO_3 \rightarrow 3KClO_4 + KCl\)
3 \(6KOH + 3Cl_2 \to 3H_2O + 5KCl + KClO_3\)
Which statement about these equations is correct?
▶️ Answer/Explanation
Ans: D
1. Disproportionation Definition: A reaction where the same element is both oxidized and reduced.
2. Equation 2: Chlorine in \(KClO_3\) (oxidation state +5) forms \(KClO_4\) (+7, oxidation) and \(KCl\) (-1, reduction).
3. Equation 3: Chlorine (\(Cl_2\), oxidation state 0) forms \(KCl\) (-1, reduction) and \(KClO_3\) (+5, oxidation).
4. Verification of Options: – A: Incorrect, \(KCl\) is not a disinfectant. – B: Incorrect, Equation 1 is a redox reaction (oxygen’s oxidation state changes). – C: Incorrect, \(KClO_4\) is chlorate(VII), not (IV). – D: Correct, both equations show disproportionation.
Topic: 12.1
Nitrogen monoxide, NO, is a primary pollutant produced by petrol engines and is found in their exhaust gases. Which reaction occurs in a catalytic converter and decreases the emission of nitrogen monoxide?
▶️ Answer/Explanation
Ans: C
In a catalytic converter, nitrogen monoxide (NO) is reduced to nitrogen gas (\(N_2\)), while carbon monoxide (CO) is oxidized to carbon dioxide (\(CO_2\)). The correct reaction is:
\[ 2NO(g) + 2CO(g) \rightarrow N_2(g) + 2CO_2(g) \]
This reaction decreases NO emissions by converting it into harmless \(N_2\) and also reduces CO levels by oxidizing it to \(CO_2\). Options A, B, and D either produce secondary pollutants (\(NO_2\)) or are chemically implausible (formation of solid carbon in a gas-phase reaction).
Topic: 13.3
The diagram shows the structure of the naturally occurring molecule cholesterol.
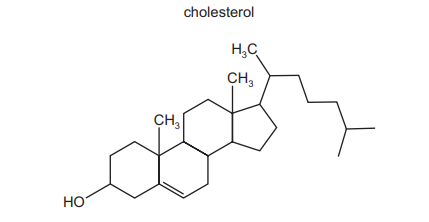
Student X stated that the 17 carbon atoms in the 4 rings all lie in the same plane. Student Y stated that this molecule displays cis/trans isomerism at the C=C double bond. Which students are correct?
▶️ Answer/Explanation
Ans: B
Analysis of Student X’s claim: The four rings in cholesterol are cyclohexane (chair conformation) and cyclopentane (envelope conformation), which are non-planar due to their 3D geometry. Thus, the 17 carbon atoms cannot lie in the same plane.
Analysis of Student Y’s claim: The C=C bond (between C5 and C6) has two identical hydrogen atoms on one carbon, making cis/trans isomerism impossible (both substituents on C5 are H atoms).
Since both statements are incorrect, the correct answer is B (neither X nor Y).
Topic: 15.1
The drug cortisone has the formula shown.
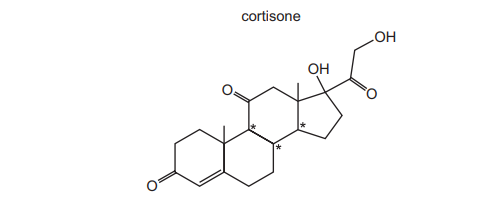
In addition to those chiral centres marked by an asterisk (*), how many other chiral centres are present in the cortisone molecule?
▶️ Answer/Explanation
Ans: D
1. A chiral centre is a carbon atom bonded to four different groups.
2. In the cortisone molecule, three additional carbons (excluding the ones marked with *) meet this criterion:
- One at the junction of the 5-membered ring and the 6-membered ring.
- Two others in the 6-membered ring where substituents create asymmetry.
3. Thus, there are 3 more chiral centres, making option D correct.
Topic: 14.2
But-2-ene reacts with cold dilute acidified KMnO₄ to give product X. But-2-ene reacts with hot concentrated acidified KMnO₄ to give product Y. Which statement about product X and product Y is correct?
A. Both product X and product Y will react with 2,4-dinitrophenylhydrazine.
B. Neither product X nor product Y will react with 2,4-dinitrophenylhydrazine.
C. Product X will react with 2,4-dinitrophenylhydrazine, product Y will not.
D. Product Y will react with 2,4-dinitrophenylhydrazine, product X will not.
▶️ Answer/Explanation
Ans: B
When but-2-ene reacts with cold dilute KMnO₄, it undergoes hydroxylation to form a diol (X), which does not contain a carbonyl group. With hot concentrated KMnO₄, it undergoes oxidative cleavage to form carboxylic acids (Y). Since 2,4-dinitrophenylhydrazine reacts only with carbonyl compounds (aldehydes/ketones), neither X (diol) nor Y (carboxylic acids) will react. Thus, the correct statement is B.
Topic: 16.1
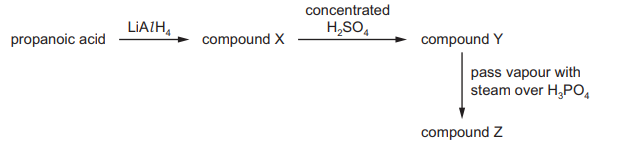
A sequence of reactions takes place. The major product is compound Z.
▶️ Answer/Explanation
Ans: D
The reaction sequence involves:
- Dehydration of propan-2-ol (secondary alcohol) to form propene (B).
- Hydration of propene (Markovnikov addition) regenerates propan-2-ol (D), not propan-1-ol (C), due to the more stable secondary carbocation intermediate.
Thus, the major product Z is propan-2-ol (D).
Topic: 17.1
Which statement is correct?
▶️ Answer/Explanation
Ans: D
- A: Incorrect. NaOH(aq) with bromoethane favors substitution (ethanol), not elimination (ethene).
- B: Incorrect. 1-bromobutane reacts faster than 1-chlorobutane (Br is a better leaving group).
- C: Incorrect. \((C_2H_5)_3CBr\) undergoes \(S_N1\) due to steric hindrance from bulky groups.
- D: Correct. The tertiary carbocation \((CH₃)₃C⁺\) is more stable than the primary \(CH₃CH₂CH₂CH₂⁺\) (hyperconjugation and inductive effects).
Topic: 17.1
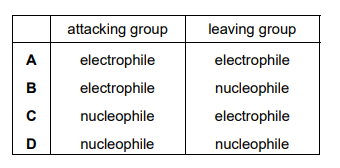
In the hydrolysis of bromoethane by aqueous NaOH, what is the nature of the attacking group and of the leaving group?
▶️ Answer/Explanation
Ans: D
In the hydrolysis of bromoethane (\(C_2H_5Br\)) with aqueous NaOH:
- The attacking group is the hydroxide ion (\(OH^-\)), which is a nucleophile (electron-rich species that donates a pair of electrons).
- The leaving group is the bromide ion (\(Br^-\)), which is a good leaving group due to its weak basicity and large size.
Thus, the correct option is D (Nucleophile for \(OH^-\) and Leaving group for \(Br^-\)).
Topic: 19.1
X is an organic compound containing the elements carbon, hydrogen and oxygen only. The table shows the observations made from three chemical tests carried out on X.
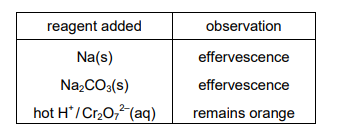
What is a possible structure of X? 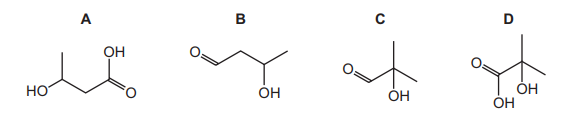
▶️ Answer/Explanation
Ans: D
The test results indicate that compound X is likely an aldehyde:
1. Tollens’ test (silver mirror) confirms an aldehyde group (–CHO).
2. 2,4-DNPH (orange precipitate) further supports the presence of a carbonyl group (C=O).
3. No effervescence with NaHCO₃ rules out carboxylic acids (–COOH).
Among the options, D (CH₃CH₂CHO, propanal) fits all observations, as it is an aldehyde without acidic protons.
Topic: 14.1
How many moles of oxygen gas are needed for the complete combustion of 1mol of (CH₃)₃COH?
▶️ Answer/Explanation
Ans: A
Balanced Combustion Equation: \[ (CH_3)_3COH + 6O_2 \to 4CO_2 + 5H_2O \] – The molecular formula of tertiary butanol, \((CH_3)_3COH\), contains **4 carbon atoms** and **10 hydrogen atoms**. – For complete combustion, each carbon requires 1 \(O_2\) to form \(CO_2\), and every 4 hydrogen atoms require 1 \(O_2\) to form \(H_2O\). – Total \(O_2\) needed: \(4 + \frac{10}{4} = 6.5\) moles. However, since \(O_2\) is diatomic, the balanced equation simplifies to **6 moles of \(O_2\)** per mole of \((CH_3)_3COH\).
Thus, the correct answer is A (6).
Topic: 19.2
In which pair will each compound give a different visible result with alkaline I₂(aq)?
A. CH₃CH₂OH and CH₃CHO
B. \(CH_3CHO\) and \(CH_3COCH_3\)
C. \(CH_3COOH\) and \(C_2H_5COC_2H_5\)
D. \(CH_3CH_2OH\) and \(C_2H_5CHO\)
▶️ Answer/Explanation
Ans: D
Alkaline \(I_2(aq)\) (iodine in sodium hydroxide solution) is used to distinguish between compounds based on their functional groups:
- Ethanol (\(CH_3CH_2OH\)): Does not react with \(I_2(aq)\), so the solution remains brown.
- Ethanal (\(C_2H_5CHO\)): Undergoes the iodoform reaction, producing a yellow precipitate of \(CHI_3\) (iodoform).
Since these two compounds give different visible results (no change vs. yellow precipitate), the correct pair is D.
Topic: 19.2
Which reagent gives a positive result with propanone?
▶️ Answer/Explanation
Ans: A
1. Iodoform Test (Alkaline \(I_2\)): Propanone (a methyl ketone) reacts with alkaline \(I_2\) to form a yellow precipitate of iodoform (\(CHI_3\)), giving a positive test. 2. Other Reagents: – B (Aqueous bromine): No reaction with propanone. – C (Fehling’s) and D (Tollens’): Only react with aldehydes, not ketones like propanone. Thus, only alkaline \(I_2\)(aq) (Option A) gives a positive result.
Topic: 18.2
Esters can be hydrolysed with an aqueous alkali or an aqueous acid to form two products. The table compares the two methods. Which row is correct?
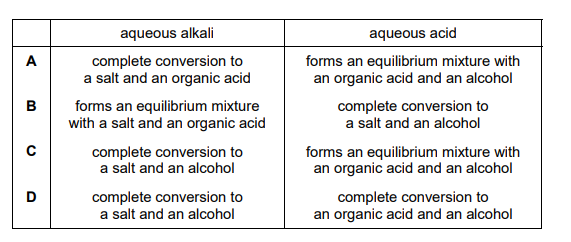
▶️ Answer/Explanation
Ans: C
Hydrolysis of esters can occur under acidic or basic conditions, with key differences:
1. Acidic Hydrolysis (Reversible):
– Products: Carboxylic acid + Alcohol
– Reaction: \(RCOOR’ + H_2O \rightleftharpoons RCOOH + R’OH\)
2. Basic Hydrolysis (Irreversible, Saponification):
– Products: Carboxylate salt + Alcohol
– Reaction: \(RCOOR’ + OH^- \rightarrow RCOO^- + R’OH\)
The table in the image shows that row C correctly states that acid hydrolysis is reversible and produces a carboxylic acid, while base hydrolysis is irreversible and produces a carboxylate salt.
Topic: 17.1
Structural isomerism only should be considered when answering this question. How many compounds with molecular formula \(C_5H_{11}Br\) are primary halogenoalkanes?
▶️ Answer/Explanation
Ans: A
To determine the number of primary bromoalkanes with formula \(C_5H_{11}Br\):
- First, identify all structural isomers of \(C_5H_{11}Br\) (8 total).
- A primary bromoalkane has the Br atom attached to a carbon that is bonded to only one other carbon.
- The four primary isomers are:
- 1-bromopentane (\(CH_3CH_2CH_2CH_2CH_2Br\))
- 2-methyl-1-bromobutane (\((CH_3)_2CHCH_2CH_2Br\))
- 3-methyl-1-bromobutane (\(CH_3CH_2CH(CH_3)CH_2Br\))
- 2,2-dimethyl-1-bromopropane (\((CH_3)_3CCH_2Br\))
Thus, there are 4 primary bromoalkanes (Option A). The other isomers are secondary or tertiary.
Topic: 17.1
Compound Z is formed by the reaction scheme shown.

What is the formula of compound Z?
▶️ Answer/Explanation
Ans: D
1. The reaction scheme shows the conversion of an alkene to a carboxylic acid through two steps: hydrohalogenation followed by hydrolysis.
2. First, \( CH_2=CH_2 \) reacts with HBr to form \( CH_3CH_2Br \).
3. The alkyl bromide then undergoes hydrolysis (reaction with water) to form \( CH_3CH_2OH \), which is further oxidized to \( CH_3CH_2COOH \) (propanoic acid).
4. Thus, the final product Z is \( CH_3CH_2COOH \), corresponding to option D.
Topic: 16.1
Hydroxyethanal, HOCH₂CHO, is heated under reflux with an excess of acidified K₂Cr₂O₇ until no further oxidation takes place. What is the skeletal formula of the organic product?
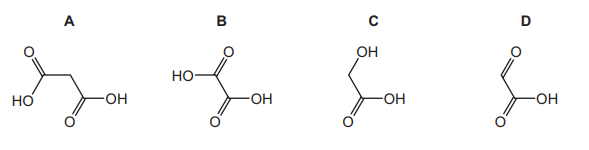
▶️ Answer/Explanation
Ans: B
Hydroxyethanal (HOCH₂CHO) contains both an alcohol (–OH) and an aldehyde (–CHO) group. Under strong oxidation with acidified K₂Cr₂O₇:
- The aldehyde group is oxidized to a carboxylic acid (–COOH).
- The primary alcohol group is also oxidized to a carboxylic acid (–COOH).
The product is ethanedioic acid (oxalic acid), with the skeletal formula B (HOOC–COOH).
Topic: 13.4
The formula shows the repeat unit of an addition polymer.
–CH(CH₃)CH(CH₂CH₃)–
What is the correct name of the monomer from which this polymer is made?
▶️ Answer/Explanation
Ans: C
To find the monomer:
- Identify the repeat unit: –CH(CH₃)CH(CH₂CH₃)–.
- Reconstruct the monomer by breaking the C–C single bond and forming a C=C double bond between the same carbons.
- This gives the monomer: CH₃–CH=CH–CH₂–CH₃ (pent-2-ene).
Option A uses incorrect IUPAC nomenclature, B is structurally incorrect, and D (pent-1-ene) would produce a different repeat unit. Thus, the correct answer is C (pent-2-ene).
Topic: 22.1
The infrared spectrum of a compound is shown.
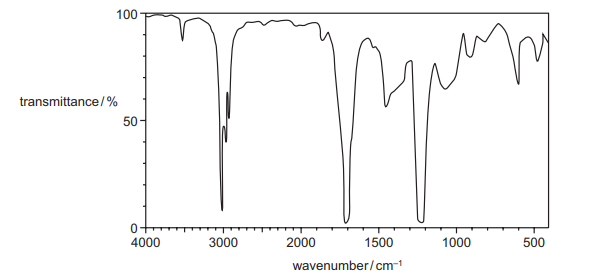
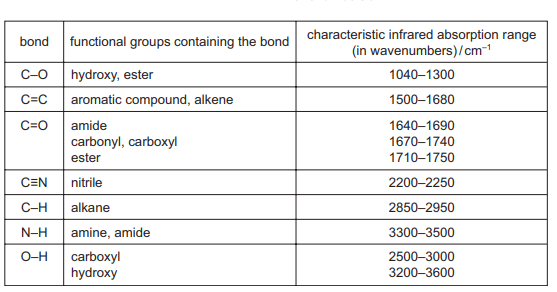
Which functional group could the compound contain?
▶️ Answer/Explanation
Ans: C
The IR spectrum shows:
- A strong absorption at ~1700 cm-1 (C=O stretch, carbonyl group).
- No broad absorption at 2500-3300 cm-1 (O-H stretch of carboxylic acid or alcohol).
- Additional peaks at ~1200-1300 cm-1 (C-O stretch, typical of esters).
These features match an ester (C=O and C-O stretches without O-H). Alcohols (A) and carboxylic acids (B) would show O-H stretches, while nitriles (D) show a sharp peak at ~2200 cm-1 (C≡N), which is absent here.
