Topic: 23.4
(a) (i) Describe the trend in the solubility of the hydroxides of magnesium, calcium and strontium. Explain your answer.

(ii) Suggest the variation in pH of saturated solutions of the hydroxides of magnesium, calcium and strontium. Explain your answer.
(b) Barium hydroxide, Ba(OH)₂, is a strong base. A 250.0 cm³ solution of Ba(OH)₂ with a pH of 12.2 is made by dissolving Ba(OH)₂ in distilled water. Calculate the mass of Ba(OH)₂ required to make this solution. Show your working.
[\(M_r: Ba(OH)_2\), 171.3]
(c) The solubility of iron(II) hydroxide, Fe(OH)₂, is 5.85 × 10⁻⁶ moldm⁻³ at 298K.
(i) Write the expression for the solubility product, \(K_{sp}\), of Fe(OH)₂.
(ii) Calculate the value of \(K_{sp}\) of Fe(OH)₂. Include its units.
▶️ Answer/Explanation
(a)(i) Solubility increases down the group (Mg(OH)₂ < Ca(OH)₂ < Sr(OH)₂).
Explanation: The solubility of Group 2 hydroxides increases down the group due to the increase in ionic radius and decrease in lattice energy. Larger ions have weaker electrostatic forces, making the lattice easier to break and the compound more soluble.
(a)(ii) pH increases down the group (Mg(OH)₂ < Ca(OH)₂ < Sr(OH)₂).
Explanation: As solubility increases, more OH⁻ ions are released into the solution, leading to higher alkalinity (pH). Thus, Sr(OH)₂ (most soluble) has the highest pH, while Mg(OH)₂ (least soluble) has the lowest.
(b) Mass of Ba(OH)₂ = 0.0215 g.
Explanation:
1. pH = 12.2 ⇒ pOH = 14 – 12.2 = 1.8 ⇒ [OH⁻] = 10⁻¹·⁸ = 0.0158 moldm⁻³.
2. Ba(OH)₂ dissociates as Ba(OH)₂ → Ba²⁺ + 2OH⁻ ⇒ [Ba(OH)₂] = [OH⁻]/2 = 0.00792 moldm⁻³.
3. Moles in 250 cm³ = 0.00792 × 0.25 = 0.00198 mol.
4. Mass = moles × \(M_r\) = 0.00198 × 171.3 = 0.0215 g.
(c)(i) \(K_{sp} = [Fe^{2+}][OH^-]^2\).
Explanation: The solubility product expression is derived from the dissociation equation Fe(OH)₂(s) ⇌ Fe²⁺(aq) + 2OH⁻(aq).
(c)(ii) \(K_{sp} = 8.01 \times 10^{-16} \, \text{mol}^3\text{dm}^{-9}\).
Explanation:
1. [Fe²⁺] = 5.85 × 10⁻⁶ moldm⁻³, [OH⁻] = 2 × 5.85 × 10⁻⁶ = 1.17 × 10⁻⁵ moldm⁻³.
2. \(K_{sp} = (5.85 \times 10^{-6}) \times (1.17 \times 10^{-5})^2 = 8.01 \times 10^{-16} \, \text{mol}^3\text{dm}^{-9}\).
Topic: 28.5
(a) (i) Define transition element.
(ii) Explain why transition elements can form complex ions.
(b) The 3d orbitals in an isolated Ag⁺ ion are degenerate.
(i) Define degenerate d orbitals.
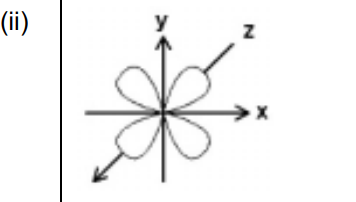
(ii) Sketch the shape of a \(3d_{xy}\) orbital in Fig. 2.1.
(c) Tollens’ reagent can be used to distinguish between aldehydes and ketones. Tollens’ reagent contains [Ag(NH₃)₂]OH, which can be prepared in a two-step process.
step 1 Aqueous NaOH is added dropwise to aqueous AgNO₃ to form Ag₂O as a brown precipitate.
step 2 Aqueous NH₃ is added dropwise to Ag₂O to form a colourless solution containing [Ag(NH₃)₂]OH.
Construct equations for each of the steps in the preparation of [Ag(NH₃)₂]OH.
(d) Name the shape of the complex ion [Ag(NH₃)₂]⁺. State the bond angle for H-N-Ag and for N-Ag-N.
bond angle for H-N-Ag = ………….. °
bond angle for N-Ag-N = ………….. °
(e) An electrochemical cell uses Ag₂O as the positive electrode and Zn as the negative electrode immersed in an alkaline electrolyte.
The overall cell reaction is shown.
\(Ag_2O + Zn + H_2O \to 2Ag + Zn(OH)_2\)
Complete the half-equation for the reaction at each electrode.
at the positive electrode Ag₂O + ……………………
at the negative electrode Zn + …………………..
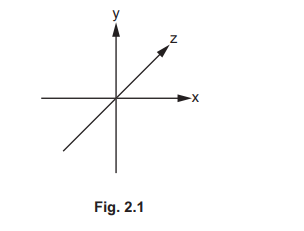
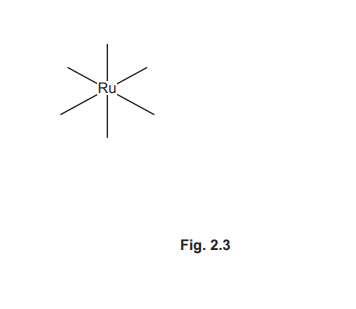
(f) Coordination polymers are made when a bidentate ligand acts as a bridge between different metal ions. Under certain conditions Ru³⁺ (aq) and the bidentate ligand dps can form a coordination polymer containing \(([Ru(dps)Cl_4]^–)_n\) chains.
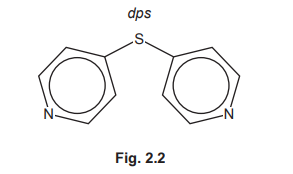
The bidentate ligand dps uses each of the nitrogen atoms to bond to a different Ru³⁺. Complete Fig. 2.3 by drawing the structure for the coordination polymer \(([Ru(dps)Cl_4]^–)_n\). Show two repeat units. The dps ligand can be represented using
▶️ Answer/Explanation
(a)(i) A transition element is defined as an element that forms one or more stable ions with incomplete d orbitals.
Explanation: Transition elements are characterized by their ability to form ions with partially filled d subshells, e.g., Fe²⁺ (3d⁶) or Cu²⁺ (3d⁹).
(a)(ii) Transition elements can form complex ions because they have vacant d orbitals that are energetically accessible for ligand bonding.
Explanation: The partially filled d orbitals allow transition metals to accept lone pairs from ligands, forming coordinate covalent bonds.
(b)(i) Degenerate d orbitals are orbitals of the same energy level (e.g., all five 3d orbitals in an isolated Ag⁺ ion).
(b)(ii) The \(3d_{xy}\) orbital has a cloverleaf shape in the xy-plane with lobes between the axes.
(c) Preparation of Tollens’ reagent:
Step 1: \(2AgNO_3 + 2NaOH \rightarrow Ag_2O + 2NaNO_3 + H_2O\)
Step 2: \(Ag_2O + 4NH_3 + H_2O \rightarrow 2[Ag(NH_3)_2]OH\)
Explanation: Silver oxide dissolves in ammonia to form the diamminesilver(I) complex.
(d) The [Ag(NH₃)₂]⁺ ion has a linear shape.
Bond angle for H-N-Ag = 180° (linear geometry).
Bond angle for N-Ag-N = 180°.
(e) Half-equations:
Positive electrode: \(Ag_2O + H_2O + 2e^- \rightarrow 2Ag + 2OH^-\)
Negative electrode: \(Zn + 2OH^- \rightarrow Zn(OH)_2 + 2e^-\)
Explanation: Ag₂O is reduced to Ag, while Zn is oxidized to Zn(OH)₂ in the alkaline electrolyte.
(f) The coordination polymer structure shows two repeat units of \([Ru(dps)Cl_4]^–\) linked via dps ligands:
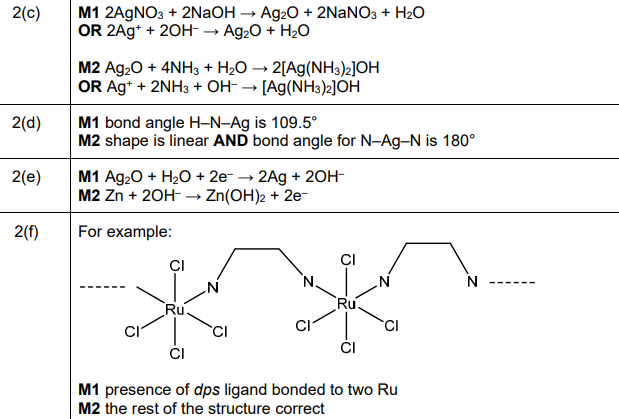
Explanation: Each Ru³⁺ is coordinated to four Cl⁻ ions and two nitrogen atoms from dps ligands, forming a chain.
Topic: 28.4
(a) When hydrated lithium ethanedioate, Li₂C₂O₄•H₂O, is heated, two gases and a white solid residue form. The residue reacts with HNO₃(aq) to produce CO₂. Complete the decomposition equation:
Li₂C₂O₄•H₂O → ……………………………… + ……………………………… + ………………………………
(b) The decomposition trend for Group 2 ethanedioates resembles that of Group 2 nitrates. Predict whether CaC₂O₄ or BaC₂O₄ decomposes at a lower temperature. Explain.
(c) Potassium iron(III) ethanedioate, K₃[Fe(C₂O₄)₃], forms a green solution in water. Explain why transition elements form coloured complexes.
(d) Anhydrous K₃[Fe(C₂O₄)₃] decomposes into K₂[Fe(C₂O₄)₂], K₂C₂O₄, and CO₂. Balance the equation:
K₃[Fe(C₂O₄)₃] → ………….. K₂[Fe(C₂O₄)₂] + ………… K₂C₂O₄ + ………… CO₂
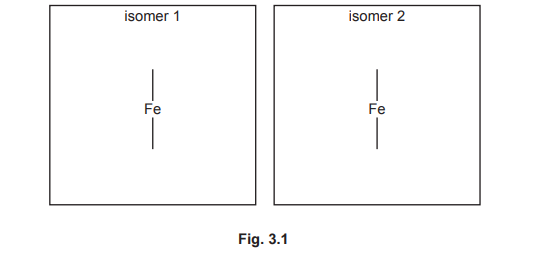
(e) The [Fe(C₂O₄)₃]³⁻ ion exhibits stereoisomerism. Complete the 3D diagrams below to show its two stereoisomers. Represent C₂O₄²⁻ as: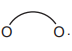
(f) Buffer solutions regulate pH. Write two equations showing how HC₂O₄⁻ acts as a buffer when acid or alkali is added.
(g) A fuel cell oxidizes ethanedioic acid in alkaline electrolyte. Given standard electrode potentials: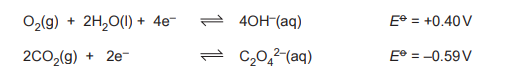
Deduce the overall cell reaction and calculate \(E^o_{cell}\).
▶️ Answer/Explanation
(a) Li₂C₂O₄•H₂O → Li₂CO₃ + CO + H₂O
Explanation: Heating decomposes the compound into lithium carbonate (white residue), carbon monoxide, and water. The residue (Li₂CO₃) reacts with HNO₃ to produce CO₂.
(b) CaC₂O₄ decomposes at a lower temperature than BaC₂O₄.
Explanation: Smaller Group 2 cations (Ca²⁺) polarize the oxalate anion more effectively, weakening the C₂O₄²⁻ bonds and lowering decomposition temperature.
(c) Transition metal complexes are coloured due to d-d electron transitions.
Explanation: In [Fe(C₂O₄)₃]³⁻, Fe³⁺ has partially filled d-orbitals. Light absorption promotes d-electrons to higher energy levels, transmitting complementary colours (green here).
(d) 2K₃[Fe(C₂O₄)₃] → 2K₂[Fe(C₂O₄)₂] + 1K₂C₂O₄ + 3CO₂
Explanation: Balancing the equation ensures conservation of Fe, K, C, and O atoms.
(e) The two stereoisomers are optical isomers (enantiomers).
Explanation: [Fe(C₂O₄)₃]³⁻ is octahedral with bidentate C₂O₄²⁻ ligands, forming non-superimposable mirror images. Diagrams show left- and right-handed configurations.
(f) Buffer action equations:
– With added acid (H⁺): HC₂O₄⁻ + H⁺ → H₂C₂O₄
– With added base (OH⁻): HC₂O₄⁻ + OH⁻ → C₂O₄²⁻ + H₂O
Explanation: HC₂O₄⁻ acts as both a weak acid (reacts with OH⁻) and weak base (reacts with H⁺), stabilizing pH.
(g) Overall reaction:
(COOH)₂ + ½O₂ → 2CO₂ + H₂O
\(E^o_{cell} = +0.46 \text{ V}\)
Explanation:
- Anode (oxidation): (COOH)₂ → 2CO₂ + 2H⁺ + 2e⁻ (\(E^o = -0.49 \text{ V}\))
- Cathode (reduction): ½O₂ + 2H⁺ + 2e⁻ → H₂O (\(E^o = +0.95 \text{ V}\))
- \(E^o_{cell} = E^o_{\text{cathode}} – E^o_{\text{anode}} = 0.95 – (-0.49) = +1.44 \text{ V}\)
Topic: 26.2
(a) Define standard electrode potential, \(E^o\), including a description of standard conditions.
(b) (i) An electrochemical cell is set up to measure \(E^o\) of the \(Ag^+\)(aq)/Ag(s) electrode. Draw a labelled diagram of this electrochemical cell. Include all necessary substances. It is not necessary to state conditions used.
(ii) A separate electrochemical cell is set up using a lower concentration of \(Ag^+\)(aq) than that used in (b)(i). Suggest how the electrode potential, E, for the \(Ag^+\)(aq)/Ag(s) electrode would change from its \(E^o\) value. Explain your answer.
(c) Define enthalpy change of solution, \(\Delta H^o_{sol}\).
(d) Some relevant energy changes for AgNO₃ are shown in Table 4.1.
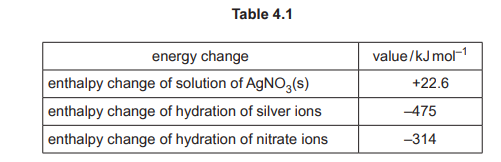
(i) Complete the energy cycle in Fig. 4.1 to show the relationship between the lattice energy, \(\Delta H^o_{latt}\), of AgNO₃(s) and the energy changes shown in Table 4.1.
Include state symbols for all the species.
(ii) Calculate the lattice energy, \(\Delta H^o_{latt}\) , of AgNO₃(s).
(e) Suggest the trend in the magnitude of the lattice energies of the metal nitrates, NaNO₃(s), Mg(NO₃)₂(s), and RbNO₃(s). Explain your answer.

▶️ Answer/Explanation
(a) Standard electrode potential (\(E^o\)) is the voltage of a half-cell compared to the standard hydrogen electrode (SHE) under standard conditions: 1 mol dm⁻³ concentration, 101 kPa pressure, and 298 K temperature.
Explanation: \(E^o\) measures the tendency of a species to gain electrons relative to SHE, with all reactants and products in their standard states.
(b) (i)
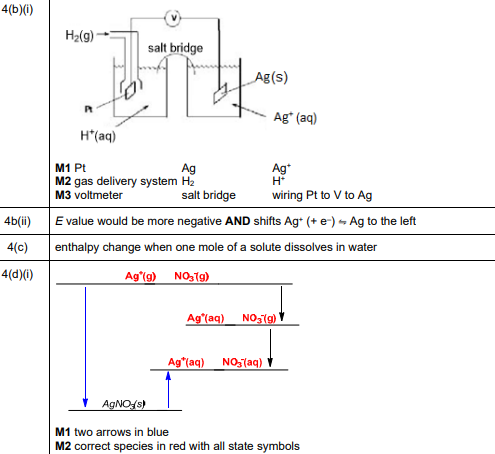
Explanation: The cell consists of an Ag electrode in 1 mol dm⁻³ \(Ag^+\)(aq) connected via a salt bridge to the SHE. A voltmeter measures the potential difference.
(b) (ii) The electrode potential \(E\) decreases from \(E^o\) because the Nernst equation predicts a lower potential for reduced \([Ag^+]\): \(E = E^o – \frac{RT}{nF} \ln \left( \frac{1}{[Ag^+]} \right)\).
Explanation: Lower \(Ag^+\) concentration reduces the driving force for reduction, decreasing the electrode potential.
(c) Enthalpy change of solution (\(\Delta H^o_{sol}\)) is the energy change when 1 mole of a solute dissolves in a solvent to form an infinitely dilute solution under standard conditions.
Explanation: It includes energy to break the lattice (\(\Delta H^o_{latt}\)) and energy released during solvation (\(\Delta H^o_{hyd}\)).
(d) (i) The completed energy cycle is:

Explanation: The cycle links \(\Delta H^o_{latt}\) (solid → gaseous ions) with \(\Delta H^o_{sol}\) (solid → aqueous ions) and \(\Delta H^o_{hyd}\) (gaseous ions → aqueous ions).
(d) (ii) \(\Delta H^o_{latt} = \Delta H^o_{sol} – \Delta H^o_{hyd} = 22.6 – ( -466.5 + (-318.5) ) = -811.6 \, \text{kJ mol}^{-1}\).
Explanation: Lattice energy is calculated using Hess’s Law: \(\Delta H^o_{latt} = \Delta H^o_{sol} – (\Delta H^o_{hyd}(Ag^+) + \Delta H^o_{hyd}(NO_3^-))\).
(e) The trend is Mg(NO₃)₂(s) > NaNO₃(s) > RbNO₃(s).
Explanation: – \(Mg^{2+}\) has a higher charge density than \(Na^+\) or \(Rb^+\), increasing lattice energy. – \(Na^+\) is smaller than \(Rb^+\), leading to stronger ionic bonding in NaNO₃ compared to RbNO₃.

Topic: 25.2
In aqueous solution, persulfate ions, \(S_2O_8^{2–}\), react with iodide ions, as shown in reaction 1.
reaction 1 \(S_2O_8^{2–} + 2I^– \to 2SO_4^{2–} + I_2\)
The rate of reaction 1 is investigated. A sample of \(S_2O_8^{2–}\) is mixed with a large excess of iodide ions of known concentration. The graph in Fig. 5.1 shows the results obtained.
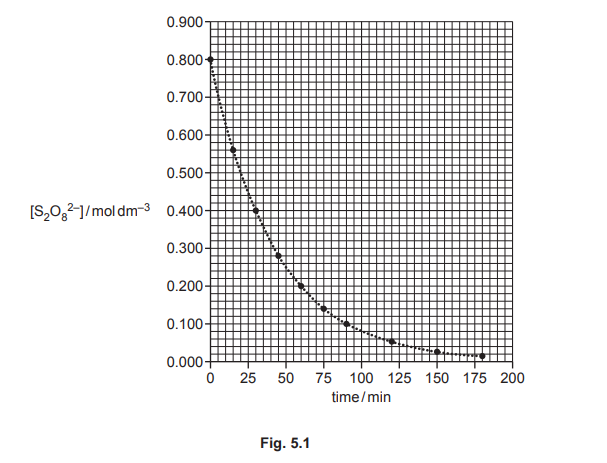
(i) Use Fig. 5.1 to determine the initial rate of reaction 1. Show your working.
(ii) The rate equation for reaction 1 is rate = \(k [S_2O_8^{2–}] [I^–]\). Suggest why a large excess of iodide ions allows the rate constant to be determined from the half-life in this investigation.
(b) The reaction of persulfate ions, \(S_2O_8^{2–}\), with iodide ions is catalysed by \(Fe^{2+}\) ions. Write two equations to show how \(Fe^{2+}\) catalyses reaction 1.
(c) Describe the effect of an increase in temperature on the rate constant and the rate of reaction 1.
(d) In aqueous solution, thiosulfate ions, \(S_2O_3^{2–}\), react with hydrogen ions, as shown in reaction 2.
reaction 2 \(S_2O_3^{2–} + 2H^+ \to SO_2 + S + H_2O\)
The rate of reaction is first order with respect to \([S_2O_3^{2–}]\) and zero order with respect to \([H^+]\) under certain conditions. The rate constant, k, for this reaction is \(1.58 \times 10^{–2} s^{–1}\). Calculate the half-life, \(t_{\frac{1}{2}}\), for reaction 2.
(e) The compound nitrosyl bromide, NOBr, can be formed as shown in reaction 3.
reaction 3 \(2NO(g) + Br_2(g) \to 2NOBr(g)\)
The rate is first order with respect to [NO] and first order with respect to \([Br_2]\). The reaction mechanism has two steps. Suggest equations for the two steps of this mechanism. State which is the rate-determining step.
▶️ Answer/Explanation
(a)(i) The initial rate is determined by drawing a tangent at \(t = 0\) on the graph and calculating its gradient. From Fig. 5.1, the gradient is approximately 0.028 mol dm⁻³ s⁻¹.
Explanation: The tangent’s slope at \(t = 0\) gives the initial rate, as rate = \(-\frac{d[S_2O_8^{2–}]}{dt}\). The calculated value falls within the range 0.016–0.040 mol dm⁻³ s⁻¹.
(a)(ii) With a large excess of \(I^–\), its concentration remains effectively constant, simplifying the rate equation to pseudo-first order: rate = \(k’ [S_2O_8^{2–}]\), where \(k’ = k[I^–]\).
Explanation: The half-life of a first-order reaction depends only on \(k’\), allowing \(k\) to be derived if \([I^–]\) is known.
(b)
Equation 1: \(2Fe^{2+} + S_2O_8^{2–} \to 2Fe^{3+} + 2SO_4^{2–}\)
Equation 2: \(2Fe^{3+} + 2I^– \to 2Fe^{2+} + I_2\)
Explanation: \(Fe^{2+}\) is oxidised by \(S_2O_8^{2–}\) in step 1 and reduced back by \(I^–\) in step 2, regenerating the catalyst.
(c) Increasing temperature raises the rate constant (\(k\)) and thus the reaction rate, as more molecules possess energy ≥ activation energy.
Explanation: The Arrhenius equation \(k = Ae^{-E_a/RT}\) shows \(k\) increases exponentially with temperature.
(d) For a first-order reaction, \(t_{1/2} = \frac{0.693}{k} = \frac{0.693}{0.0158} \approx 43.9 \text{ s}\).
Explanation: The half-life is independent of concentration for first-order reactions, calculated directly from \(k\).
(e)
Step 1 (slow/RDS): \(NO + Br_2 \to NOBr_2\)
Step 2 (fast): \(NOBr_2 + NO \to 2NOBr\)
Rate-determining step: Step 1
Explanation: The rate law (first order in both NO and Br₂) matches the stoichiometry of the slow step, confirming it as the RDS.
Topic: 29.4
(a) (i) State what is meant by partition coefficient, \(K_{pc}\).
(ii) The partition coefficient, \(K_{pc}\), for a compound, X, between carbon disulfide, CS₂, and water is 10.5. 1.85 g of X is dissolved in water and made up to 100.0 cm³ in a volumetric flask. 40.0 cm³ of this aqueous solution is shaken with 25.0 cm³ of CS₂. The mixture is left to reach equilibrium. Calculate the mass of X, in g, extracted into the CS₂ layer.
(b) The compound \(C_6H_6\) has many structural isomers. Four suggested structures of \(C_6H_6\) are shown in Fig. 6.1.
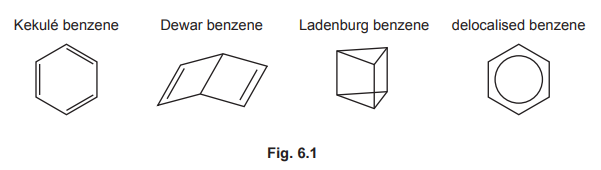
Using Fig. 6.1, complete Table 6.1 to predict the number of carbon atoms that have sp, \(sp^2\), and \(sp^3\) hybridisation in Kekulé benzene, Dewar benzene and Ladenburg benzene. 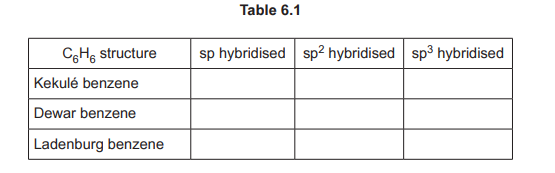
(c) Describe the shape of delocalised benzene. Include the geometry of each carbon, the C-C-H bond angle and the type of bond(s) between the carbon atoms and between the carbon and hydrogen atoms.
(d) Suggest why Dewar benzene and Ladenburg benzene are unstable isomers of \(C_6H_6\).
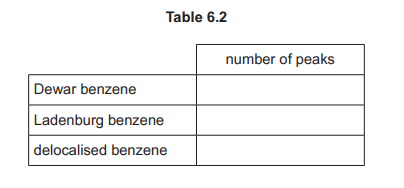
(e) Complete Table 6.2 to predict the number of peaks in the proton \((^1H)\) NMR spectrum for Dewar benzene, Ladenburg benzene and delocalised benzene.
(f) The reaction of phenylethanone with 1,4-dibromobutane, BrCH₂CH₂CH₂CH₂Br, in the presence of FeBr₃ is shown in Fig. 6.2.
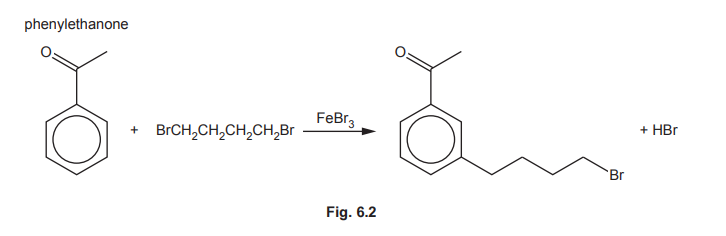 The mechanism of this reaction is similar to that of the alkylation of benzene.
The mechanism of this reaction is similar to that of the alkylation of benzene.
(i) Construct an equation for the formation of the electrophile, BrCH₂CH₂CH₂CH₂⁺.
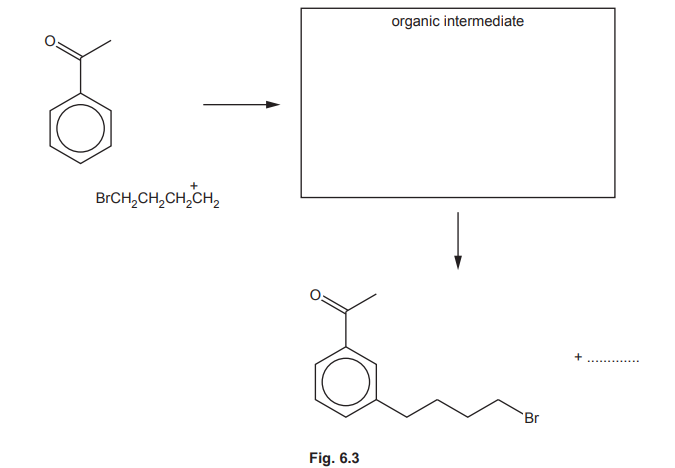
(ii) Complete the mechanism in Fig. 6.3 for the reaction of phenylethanone with BrCH₂CH₂CH₂CH₂⁺ ions. Include all relevant curly arrows and charges. Draw the structure of the organic intermediate.
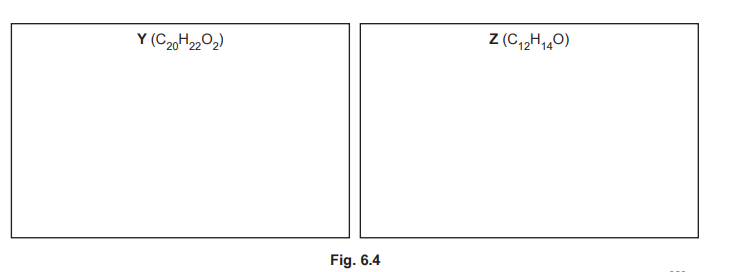
(iii) The reaction shown in Fig. 6.2 forms small amounts of two by-products, Y \((C_{20}H_{22}O_2)\) and Z \((C_{12}H_{14}O)\). Suggest structures for Y and Z in the boxes in Fig. 6.4.
▶️ Answer/Explanation
(a)(i) The partition coefficient (\(K_{pc}\)) is the ratio of concentrations of a solute in two immiscible solvents at equilibrium.
(a)(ii) Mass of X extracted into CS₂ = 0.642 g
Explanation:
1. Initial mass in 40.0 cm³ aqueous solution: \(\frac{1.85}{100} \times 40 = 0.74 \text{ g}\)
2. Let \(y\) = mass in CS₂. Then \(\frac{y/25}{(0.74 – y)/40} = 10.5\)
3. Solving gives \(y = 0.642 \text{ g}\)
(b) Hybridisation in C₆H₆ isomers:
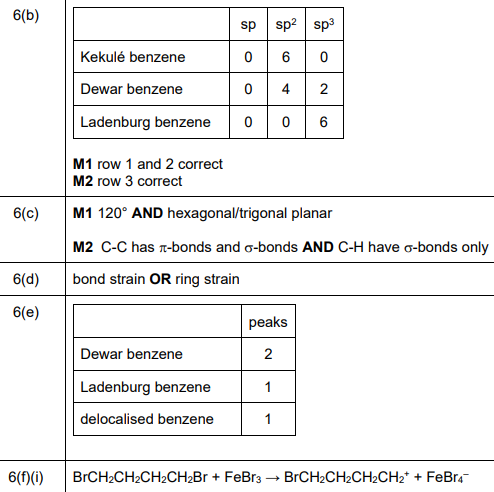
Explanation:
– Kekulé benzene: 6 \(sp^2\) carbons (planar, 120°)
– Dewar benzene: 4 \(sp^3\) (tetrahedral) + 2 \(sp^2\)
– Ladenburg benzene: 3 \(sp^3\) + 3 \(sp^2\)
(c) Delocalised benzene:
– Planar hexagonal ring with 6 \(sp^2\) hybridised carbons
– C-C-H bond angle: 120°
– C-C bonds: delocalised π-system (1.5 bond order)
– C-H bonds: σ bonds
(d) Instability of Dewar/Ladenburg benzene:
– High angle strain in small rings (Dewar)
– Loss of aromatic stabilisation (both)
– Torsional strain (Ladenburg)
(e) \(^1H\) NMR peaks:
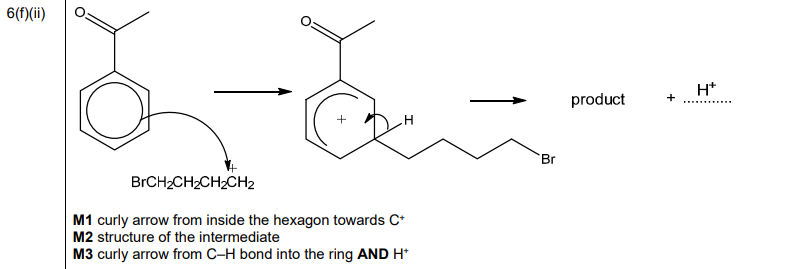
Explanation:
– Delocalised benzene: 1 peak (all H equivalent)
– Dewar: 3 peaks (different H environments)
– Ladenburg: 4 peaks
(f)(i) Electrophile formation:
\(\text{BrCH}_2\text{CH}_2\text{CH}_2\text{CH}_2\text{Br} + \text{FeBr}_3 \rightarrow \text{BrCH}_2\text{CH}_2\text{CH}_2\text{CH}_2^+ + \text{FeBr}_4^-\)
(f)(ii) Mechanism:
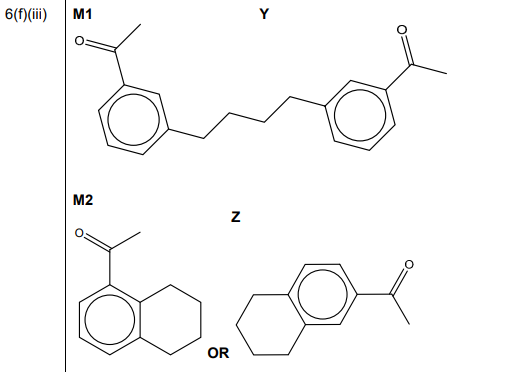
Key steps:
1. Curly arrow from benzene ring to electrophile
2. Formation of arenium ion intermediate
3. Deprotonation to restore aromaticity
(f)(iii) By-products:
– Y: Dimer of two phenylethanone units bridged by butyl chains
– Z: Monobutylated phenylethanone
Topic: 37.3
Four esters, A, B, C and D, with the molecular formula \(C_6H_{12}O_2\) are shown in Fig. 7.1.
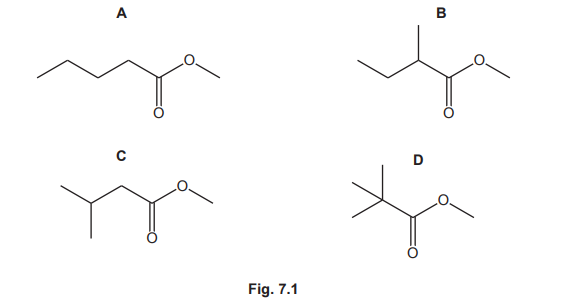
(a) Give the systematic name of ester A.
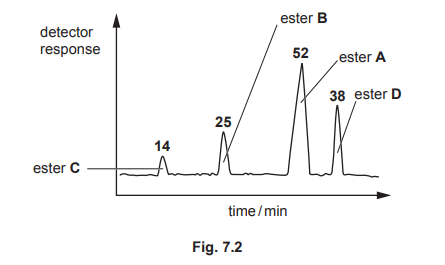
(b) A mixture of these esters, A, B, C and D, is analysed by gas–liquid chromatography. The chromatogram produced is shown in Fig. 7.2. The number above each peak represents the area under the peak. The area under each peak is proportional to the mass of the respective ester in the mixture.
(i) State what is meant by retention time.
(ii) Calculate the percentage by mass of ester D in the original mixture.
(c) Separate samples of the esters, A, B, C and D, are analysed using proton \((^1H)\) NMR and carbon-13 NMR spectroscopy.
(i) Complete Table 7.1 to show the number of peaks in each NMR spectrum for esters B and C.
(ii) Identify all of the esters from A, B, C and D that have at least one triplet peak in their proton \((^1H)\) NMR spectrum.
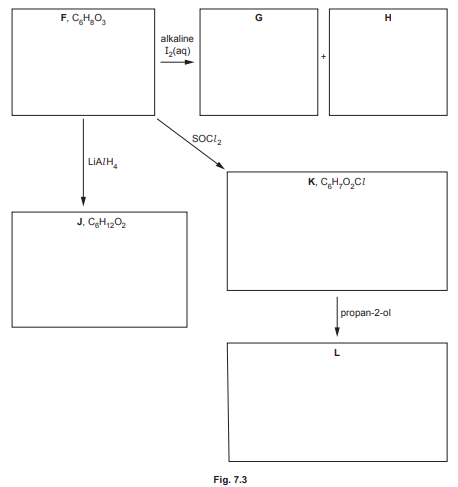
(d) Compound F, \(C_6H_8O_3\), shows stereoisomerism and effervesces with \(Na_2CO_3(aq)\).
Compound F reacts with alkaline \(I_2(aq)\) to form yellow precipitate G and compound H.
Compound F reacts with \(LiAlH_4\) to form compound J, \(C_6H_{12}O_2\).
Compound F reacts with SOCl₂ to form compound K, C₆H₇O₂Cl.
Compound K reacts with propan-2-ol to form compound L.
Draw the structures of compounds F, G, H, J, K and L in the boxes in Fig. 7.3.
▶️ Answer/Explanation
(a) The systematic name of ester A is methyl pentanoate.
Explanation: A is formed from methanol (methyl) and pentanoic acid (pentanoate), giving the IUPAC name methyl pentanoate.
(b)(i) Retention time is the time taken for a compound to travel from the injection point to the detector in gas chromatography.
Explanation: It is characteristic of each compound under specific conditions.
(b)(ii) The percentage by mass of ester D is 29.5%.
Explanation: The area under peak D is 59, and the total area is 200 (45 + 56 + 40 + 59). Thus, %D = (59/200) × 100 = 29.5%.
(c)(i) The completed Table 7.1 is:
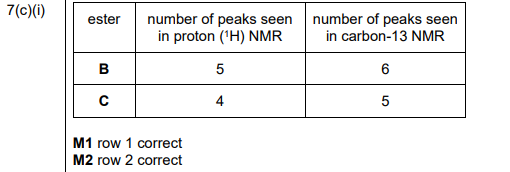
Explanation:
- Ester B: 3 peaks in \(^1H\) NMR (due to 3 proton environments) and 4 peaks in \(^{13}C\) NMR (4 carbon environments).
- Ester C: 2 peaks in \(^1H\) NMR (2 proton environments) and 3 peaks in \(^{13}C\) NMR (3 carbon environments).
(c)(ii) Esters A and B have at least one triplet peak in their \(^1H\) NMR spectrum.
Explanation: Triplets arise from protons adjacent to \(-CH_2-\) groups (e.g., \(-OCH_2CH_3\) in A and B).
(d) The structures of compounds F, G, H, J, K, and L are:
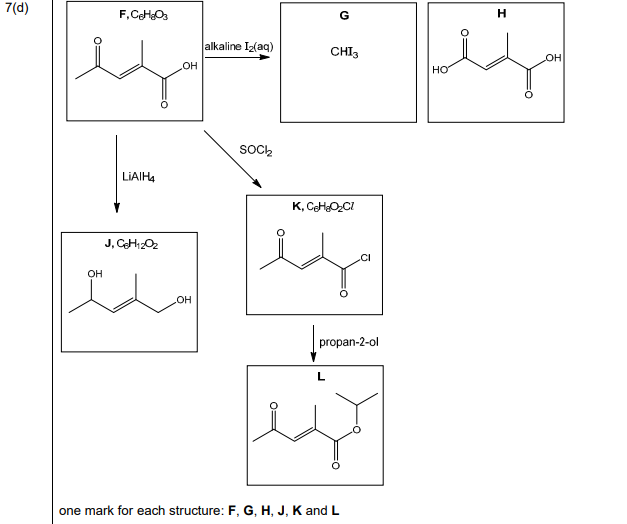
Explanation:
- F: Stereoisomeric compound with \(-COOH\) (effervesces with \(Na_2CO_3\)) and \(-COCH_3\) (reacts with \(I_2\) to form iodoform, G).
- G: Yellow precipitate is iodoform (\(CHI_3\)).
- H: Sodium salt of the acid formed after iodoform reaction.
- J: Reduction product (\(C_6H_{12}O_2\)) from \(LiAlH_4\).
- K: Acyl chloride formed with SOCl₂.
- L: Ester formed from K and propan-2-ol.
Topic: 35.2
Neotame is an artificial sweetener added to some foods.
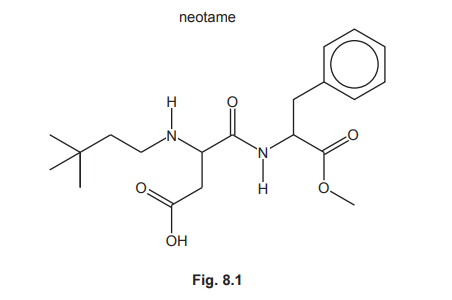
(a) (i) State the number of chiral carbon atoms in a molecule of neotame.
(ii) Neotame contains the arene functional group. Identify all the other functional groups present in neotame.
(b) Neotame reacts with an excess of hot NaOH(aq) to form three organic products.
(i) State the two types of reaction that occur when neotame reacts with hot NaOH(aq).
(ii) Draw the structures of the three organic products formed from the reaction of neotame with an excess of hot NaOH(aq). 
▶️ Answer/Explanation
(a)(i)
Answer: 2
Explanation: Neotame has two chiral centers – the carbon atoms bonded to the ester group (1) and the amide nitrogen (2), each with four different substituents.
(a)(ii)
Answer: Amide, amine, ester, carboxylic acid
Explanation: The functional groups are:
- Amide (–CONH–) at the center
- Amine (–NH2) on the right branch
- Ester (–COO–) on the left branch
- Carboxylic acid (–COOH) at the top
(b)(i)
Answer: Hydrolysis and acid-base reaction
Explanation:
- Hydrolysis: The ester and amide groups undergo base hydrolysis (saponification)
- Acid-base: The carboxylic acid group deprotonates in NaOH
(b)(ii)
Answer: 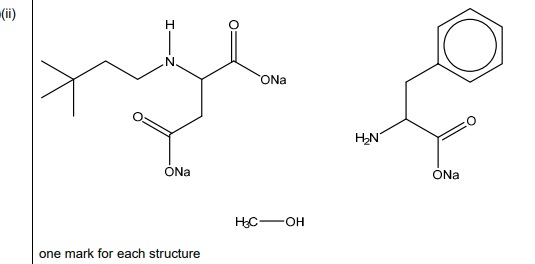
Explanation: The three products are:
- Benzoic acid (from ester hydrolysis)
- Aspartic acid derivative (from amide hydrolysis)
- Methanol (from ester hydrolysis)
The image shows the complete structures of these products.
Topic: 29.4
(a) Samples of phenol, C₆H₅OH, are reacted separately with sodium and with dilute nitric acid.
(i) Write the equation for the reaction of C₆H₅OH with Na.
(ii) Draw the structures of the two major isomeric organic products formed in the reaction of phenol with dilute HNO₃.
(b) Salicylic acid can be synthesised from phenol.
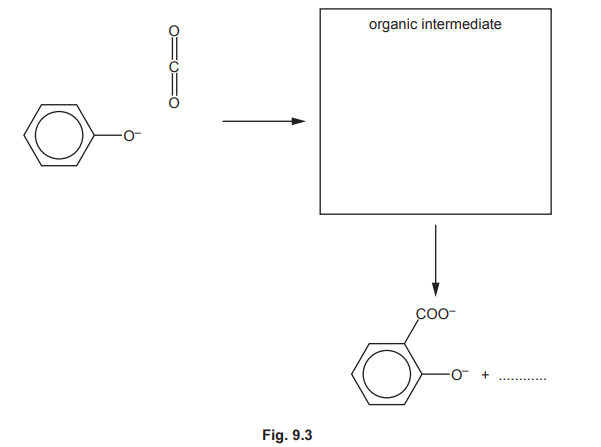
One of the steps in this synthesis is the electrophilic substitution reaction of carbon dioxide with the phenoxide ion, C₆H₅O⁻. Complete the mechanism in Fig. 9.3 for the reaction of C₆H₅O⁻ with \(CO_2\). Include all relevant curly arrows, dipoles and charges. Draw the structure of the organic intermediate.
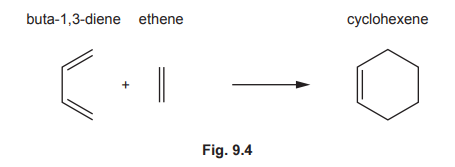
(c) Some syntheses use Diels–Alder reactions, which normally involve a diene and an alkene reacting together to form a cyclohexene.
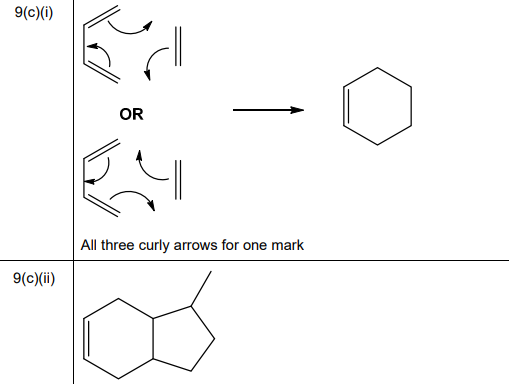
(i) Draw three curly arrows in Fig. 9.4 to complete the mechanism for the Diels–Alder reaction between buta-1,3-diene and ethene.
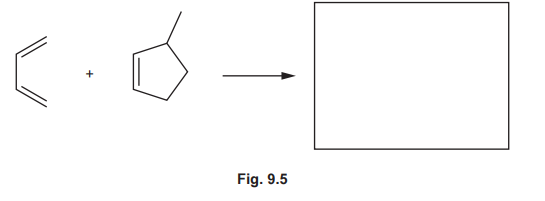
(ii) Another Diels–Alder reaction of buta-1,3-diene is shown in Fig. 9.5. Predict the product formed in this reaction.
▶️ Answer/Explanation
(a)(i) \(2C_6H_5OH + 2Na \rightarrow 2C_6H_5O^-Na^+ + H_2\)
Explanation: Phenol reacts with sodium to form sodium phenoxide (\(C_6H_5O^-Na^+\)) and hydrogen gas, similar to alcohols.
(a)(ii) Major products: 2-nitrophenol and 4-nitrophenol.
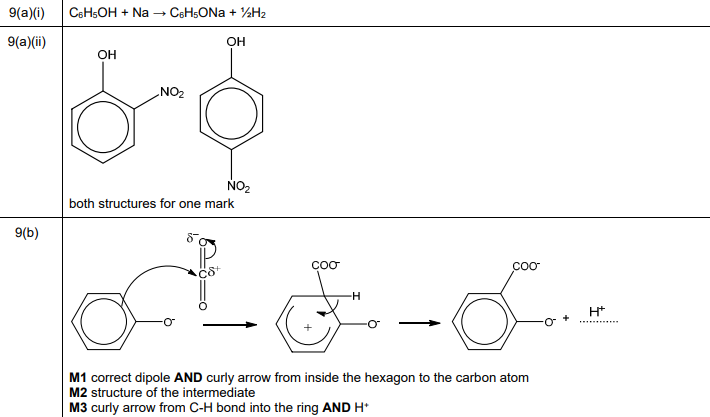
Explanation: Nitration of phenol with dilute HNO₃ preferentially substitutes at the ortho (2-) and para (4-) positions due to the \(-OH\) group’s activating and directing effects.
(b) Mechanism for reaction of phenoxide ion (\(C_6H_5O^-\)) with \(CO_2\):
Explanation:
- Nucleophilic attack by phenoxide on the electrophilic carbon of \(CO_2\).
- Formation of a carboxylate intermediate (salicylate ion).
- Protonation yields salicylic acid.
(c)(i) Diels-Alder mechanism (buta-1,3-diene + ethene):

Explanation: Three curly arrows show the concerted formation of two new σ-bonds and one π-bond, resulting in cyclohexene.
(c)(ii) Product of buta-1,3-diene with maleic anhydride:

Explanation: The reaction yields a cyclohexene derivative with an anhydride group, retaining stereochemistry of the dienophile (cis).
