Topic: 1.1
Which particle contains 8 protons, 9 neutrons and 10 electrons?
▶️ Answer/Explanation
Ans: D
The particle has 8 protons (atomic number = 8, oxygen), 9 neutrons (mass number = 8 + 9 = 17), and 10 electrons (charge = 2- due to 2 extra electrons). Thus, the correct notation is \(_8^{17}O^{2-}\), which matches option D. Options A and C have incorrect charges, and option B has the wrong mass number.
Topic: 1.3
The second ionisation energy of oxygen is greater than the second ionisation energy of fluorine. Which factor explains this difference?
▶️ Answer/Explanation
Ans: C
The second ionisation energy of oxygen is higher than fluorine because, in fluorine, the second electron is removed from a spin-paired \( 2p \) orbital, which experiences electron-electron repulsion, making it easier to remove. In oxygen, the second electron is removed from a singly occupied orbital, which requires more energy due to greater nuclear attraction. Thus, option C is correct.
Topic: 4.2
Zinc reacts with concentrated nitric acid giving three products only: zinc nitrate, an oxide of nitrogen and water. 3.0 moles of zinc react with 8.0 moles of nitric acid. Zinc nitrate contains Zn²⁺ ions. What could be the formula of the oxide of nitrogen?
▶️ Answer/Explanation
Ans: B
Balancing the reaction: \[ 3Zn + 8HNO_3 \rightarrow 3Zn(NO_3)_2 + 2NO + 4H_2O \] For 3 moles of Zn, 6 moles of \(HNO_3\) form \(Zn(NO_3)_2\), leaving 2 moles of \(HNO_3\) to produce the nitrogen oxide. The only oxide fitting this stoichiometry is **NO**, as it requires 2 moles of \(HNO_3\) per mole of NO (option B).
Topic: 1.4
A 3.7 g sample of copper(II) carbonate is added to 25 cm³ of 2.0 mol dm⁻³ hydrochloric acid. Which volume of gas is produced at room conditions?
▶️ Answer/Explanation
Ans: A
The reaction is: \(\text{CuCO}_3 + 2\text{HCl} \rightarrow \text{CuCl}_2 + \text{H}_2\text{O} + \text{CO}_2\). Moles of \(\text{CuCO}_3 = \frac{3.7}{123.5} = 0.03 \, \text{mol}\). Moles of \(\text{HCl} = 2.0 \times 0.025 = 0.05 \, \text{mol}\). \(\text{HCl}\) is limiting, producing \(0.025 \, \text{mol} \, \text{CO}_2\). Volume of gas \(= 0.025 \times 24 = 0.60 \, \text{dm}^3\) (at room conditions).
Topic: 3.4
Ammonium ions, NH₄⁺, are formed when ammonia gas reacts with hydrogen chloride gas. Which statement about the changes that occur in this reaction is correct?
▶️ Answer/Explanation
Ans: C
In the formation of NH₄⁺, nitrogen in ammonia (NH₃) undergoes a change from three bonds and one lone pair (sp³ hybridisation) to four bonds (still sp³ hybridisation). The hybridisation remains the same, making option C correct. The H−N−H bond angle increases (not decreases) in NH₄⁺ due to the absence of a lone pair, and electron transfer occurs from ammonia to hydrogen chloride, not nitrogen to chlorine.
Topic: 14.2
Which feature is present in both ethene and poly(ethene)?
▶️ Answer/Explanation
Ans: C
Ethene (C2H4) has a double bond consisting of 1 σ bond and 1 π bond, while poly(ethene) has only σ bonds in its saturated carbon chain. The common feature is the presence of σ covalent bonds in both. Bond angles (120° in ethene, ~109.5° in poly(ethene)), π bonds (absent in poly(ethene)), and sp3 orbitals (only in poly(ethene)) are not shared.
Topic: 3.2
Two compounds of boron are sodium borohydride, NaBH₄, and boron trifluoride, BF₃. What are the shapes of the borohydride ion and the boron trifluoride molecule?
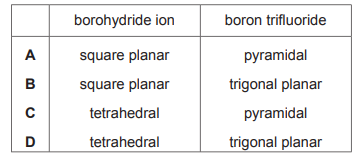
▶️ Answer/Explanation
Ans: D
The borohydride ion (BH₄⁻) has a tetrahedral shape because boron forms four single bonds (σ bonds) with hydrogen atoms, resulting in a steric number of 4. Boron trifluoride (BF₃) has a trigonal planar shape because boron forms three σ bonds with fluorine atoms and has no lone pairs, giving it a steric number of 3. Thus, the correct option is D (tetrahedral and trigonal planar).
Topic: 1.4
In an experiment, 0.100 mol of propan-1-ol is burnt completely in 12.0 dm³ of oxygen, measured at room conditions. What is the final volume of gas, measured at room conditions?
▶️ Answer/Explanation
Ans: B
The balanced equation for the combustion of propan-1-ol is:
\[ C_3H_7OH + 4.5O_2 \rightarrow 3CO_2 + 4H_2O \]
Given 0.100 mol of propan-1-ol, it reacts with 0.450 mol of \( O_2 \), producing 0.300 mol of \( CO_2 \). The remaining \( O_2 \) is \( 0.500 – 0.450 = 0.050 \) mol (since 12.0 dm³ of \( O_2 \) at room conditions is 0.500 mol). The total gas volume after combustion is \( 0.300 + 0.050 = 0.350 \) mol, which corresponds to \( 8.40 dm^3 \).
Topic: 1.2
At a temperature of 2500 K and a pressure of \(1.00 × 10^{–4} \text{ Pa}\), a sample of 0.321 g of sulfur vapour has a volume of \(2.08 × 10^6 \text{ m}^3\). What is the molecular formula of sulfur under these conditions?
▶️ Answer/Explanation
Ans: A
Using the ideal gas equation \(PV = nRT\), we calculate the number of moles (\(n\)) of sulfur. Given \(P = 1.00 × 10^{–4} \text{ Pa}\), \(V = 2.08 × 10^6 \text{ m}^3\), \(T = 2500 \text{ K}\), and \(R = 8.314 \text{ J mol}^{-1} \text{ K}^{-1}\), we find \(n ≈ 0.01 \text{ mol}\). The molar mass of sulfur is \(0.321 \text{ g} / 0.01 \text{ mol} ≈ 32.1 \text{ g/mol}\), which corresponds to the atomic mass of sulfur (S). Thus, the molecular formula is S.
Topic: 9.3
In the structure of solid SiO₂
each silicon atom is bonded to x oxygen atoms
each oxygen atom is bonded to y silicon atoms
each bond is a z type bond.
What is the correct combination of x, y and z in these statements?
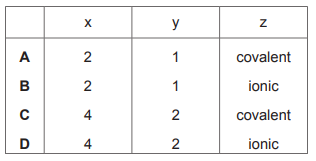
▶️ Answer/Explanation
Ans: C
In the SiO₂ structure, each silicon atom forms 4 covalent bonds with oxygen atoms (x=4), while each oxygen atom bridges between 2 silicon atoms (y=2). The bonds are covalent (z=covalent) as SiO₂ forms a giant covalent structure. This matches combination C in the given options.
Topic: 5.1
Nitric acid is made industrially by the oxidation of ammonia. The overall equation for the process is shown.
equation 1 \(NH_3 + 2O_2 → HNO_3 + H_2O\)
The process happens in three stages. The equations and enthalpy changes for these stages are given.
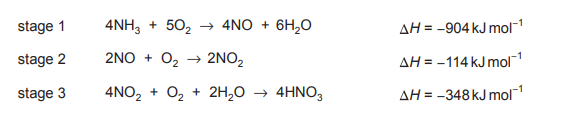 What is the enthalpy change of the process shown in equation 1?
What is the enthalpy change of the process shown in equation 1?
A. −1480 kJ mol⁻¹
B. −370 kJ mol⁻¹
C. −341.5 kJ mol⁻¹
D. +82 kJ mol⁻¹
▶️ Answer/Explanation
Ans: B
The enthalpy change for the overall reaction (equation 1) is the sum of the enthalpy changes of the three given stages. Using Hess’s Law, we add the enthalpies: \(-1168\ \text{kJ mol}^{-1} + (-114\ \text{kJ mol}^{-1}) + (-348\ \text{kJ mol}^{-1}) = -1630\ \text{kJ mol}^{-1}\). However, the overall reaction is half of the combined stages, so we divide by 4 (due to stoichiometric adjustments) to get \(\Delta H = -370\ \text{kJ mol}^{-1}\), making option B correct.
Topic: 6.1
Chlorine reacts with sodium bromide.
Cl₂ + NaBr → NaCl + Br₂
Which words correctly describe this reaction?
1 redox
2 displacement
3 disproportionation
▶️ Answer/Explanation
Ans: B
1. Redox: Chlorine (Cl₂) is reduced to Cl⁻ (oxidation number decreases from 0 to -1), while bromide (Br⁻) is oxidized to Br₂ (oxidation number increases from -1 to 0).
2. Displacement: Chlorine displaces bromine from NaBr, forming NaCl and Br₂.
3. Not disproportionation: Disproportionation requires the same element to be both oxidized and reduced, which does not occur here.
Thus, the correct descriptions are 1 and 2 only (Option B).
Topic: 6.1
The equation for the reaction between aqueous copper ions and aqueous iodide ions is as follows.
\(2Cu^{2+}(aq) + 4I^–(aq) → 2CuI(s) + I_2(aq)\)
What is the change in oxidation state of copper?
▶️ Answer/Explanation
Ans: C
In the reaction, copper starts as \( Cu^{2+} \) (oxidation state +2) and forms \( CuI \). In \( CuI \), iodine has an oxidation state of –1, so copper must be +1 to balance the charge. Thus, the oxidation state of copper changes from +2 to +1, making option C correct.
Topic: 1.2
A mixture of the three gases, oxygen, nitrogen and argon, is at a total pressure of 500 kPa. There is a total of 1.2 moles of gas in the mixture. If the oxygen gas alone occupied the entire volume of the mixture, it would exert a pressure of 150 kPa. At room conditions the amount of nitrogen gas in the mixture would occupy a volume of 5.76 dm³. What is the partial pressure of the argon gas in the mixture?
▶️ Answer/Explanation
Ans: C
1. **Partial Pressure of Oxygen (\(P_{O_2}\))**: Given as **150 kPa** (if it occupied the entire volume). 2. **Moles of Nitrogen (\(n_{N_2}\))**: Using \(V = 5.76 \, \text{dm}^3\) and \(pV = nRT\) (assuming room conditions, \(V_m \approx 24 \, \text{dm}^3/\text{mol}\)), \[ n_{N_2} = \frac{5.76}{24} = 0.24 \, \text{moles} \] Its partial pressure is: \[ P_{N_2} = \left(\frac{0.24}{1.2}\right) \times 500 = 100 \, \text{kPa} \] 3. **Partial Pressure of Argon (\(P_{Ar}\))**: \[ P_{Ar} = P_{\text{total}} – P_{O_2} – P_{N_2} = 500 – 150 – 100 = 250 \, \text{kPa} \]
Topic: 8.1
0.200 mol of sulfur dioxide and 0.200 mol of oxygen are placed in a 1.00 dm³ sealed container. The gases are allowed to react until equilibrium is reached.
\(2SO_2 + O_2 \rightleftharpoons 2SO_3\)
At equilibrium there is 0.100 mol of SO₃ in the container. What is the value of \(K_c\)?
▶️ Answer/Explanation
Ans: D
The equilibrium concentrations are calculated as follows:
Initial moles: \([SO_2] = 0.200 \, \text{mol}, [O_2] = 0.200 \, \text{mol}, [SO_3] = 0 \, \text{mol}\).
At equilibrium, \([SO_3] = 0.100 \, \text{mol}\), so:
\([SO_2] = 0.200 – 0.100 = 0.100 \, \text{mol dm}^{-3}\),
\([O_2] = 0.200 – 0.050 = 0.150 \, \text{mol dm}^{-3}\) (since 2 mol \(SO_2\) reacts with 1 mol \(O_2\)).
The equilibrium constant \(K_c\) is:
\[ K_c = \frac{[SO_3]^2}{[SO_2]^2 [O_2]} = \frac{(0.100)^2}{(0.100)^2 (0.150)} = 6.67 \, \text{mol}^{-1} \, \text{dm}^3. \]
Topic: 7.1
The decomposition of hydrogen peroxide in the presence of MnO₂ produces water and oxygen gas.
\(2H_2O_2(aq) → 2H_2O(l) + O_2(g)\)
The volume of gas collected when 0.2 g of MnO₂ is added to two different hydrogen peroxide solutions at 20°C is shown on the graph as curves X and Y.
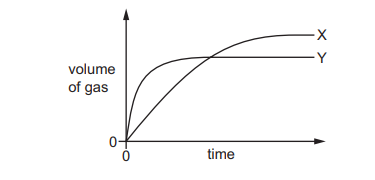
Which row shows the conditions that will result in curves X and Y? 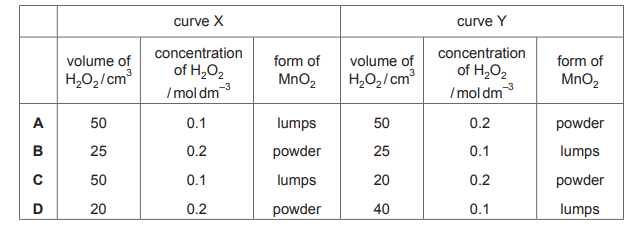
▶️ Answer/Explanation
Ans: C
The graph shows two curves, X and Y, where X produces oxygen gas faster (steeper slope) but reaches a lower total volume, while Y produces gas slower but reaches a higher volume. This indicates:
- Curve X: Higher concentration of \(H_2O_2\) (faster reaction due to more reactant particles) but smaller volume (less total \(H_2O_2\) available).
- Curve Y: Lower concentration of \(H_2O_2\) (slower reaction) but larger volume (more total \(H_2O_2\) available).
Thus, Option C (X: 50 cm³ of 2.0 mol/dm³ \(H_2O_2\); Y: 100 cm³ of 1.0 mol/dm³ \(H_2O_2\)) matches the graph.
Topic: 8.2
The diagram shows a gas syringe with a free-moving piston. The syringe contains gaseous hydrogen, gaseous iodine and gaseous hydrogen iodide at equilibrium.
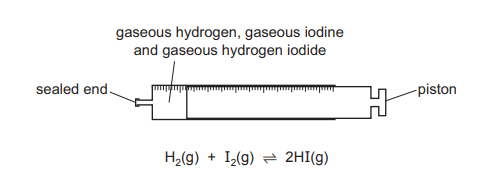
Three changes are listed.
- Increasing the total pressure by adding an inert gas and keeping the volume constant
- Increasing the pressure by adding more gaseous hydrogen iodide and keeping the volume constant
- Decreasing the volume by pushing the piston to the left
Which changes will result in an equilibrium position at which the rate of the forward reaction has increased?
▶️ Answer/Explanation
Ans: D
The equilibrium reaction is: \[ \text{H}_2(g) + \text{I}_2(g) \rightleftharpoons 2\text{HI}(g) \] Change 1 (inert gas): No effect on equilibrium (partial pressures unchanged). Change 2 (adding HI): Increases [HI], shifting equilibrium left (Le Chatelier’s principle), but the forward reaction rate increases temporarily due to higher collision frequency. Change 3 (decreasing volume): Favors the side with fewer gas moles (forward reaction, 2 moles → 2 moles), but the rate of both reactions increases due to higher pressure. Only 2 and 3 lead to an increased forward reaction rate, making D correct.
Topic: 9.2
Which row gives the best description of the variations in the melting points and the first ionisation energies of the elements in Period 3 from sodium to argon?
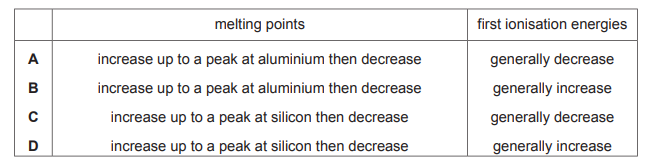
▶️ Answer/Explanation
Ans: D
Melting Points: Sodium (Na) to Aluminium (Al) increase due to stronger metallic bonding. Silicon (Si) has the highest melting point (giant covalent structure), then drops sharply for non-metals (P, S, Cl, Ar).
First Ionisation Energies: Generally increase across Period 3 (Na to Ar) due to increasing nuclear charge, but exceptions occur at Al (slightly lower than Mg) and S (slightly lower than P) due to electron shielding and repulsion effects.
Row D correctly describes both trends: melting points peak at Si, while ionisation energies show a general increase with minor deviations.
Topic: 9.1
X and Y are atoms of different elements in Period 3 of the Periodic Table. Neither X nor Y is argon. X is a non-metal. X has a greater atomic radius than Y. Which statement is correct?
▶️ Answer/Explanation
Ans: D
Since X and Y are in Period 3, they have the same number of electron shells. X is a non-metal with a larger atomic radius than Y, which means Y must have a higher effective nuclear charge (more protons) and thus a smaller radius. This implies Y is likely a metal (as metals in Period 3 have smaller radii than non-metals). However, the only correct statement among the options is D, because if Y were a non-metal, its radius would be larger than X’s, contradicting the given condition. Therefore, Y must be a metal, making option D the correct choice.
Topic: 10.1
Four mixtures are added to four separate 50 cm³ samples of water and stirred. Which mixture results in a solution with the highest pH?
▶️ Answer/Explanation
Ans: B
Magnesium oxide (\(MgO\)) is a basic oxide that reacts with water to form magnesium hydroxide (\(Mg(OH)_2\)), increasing the pH. In contrast, phosphorus oxide (\(P_4O_{10}\)) and silicon dioxide (\(SiO_2\)) are acidic oxides, while aluminium oxide (\(Al_2O_3\)) is amphoteric. Since \(MgO\) is the only strongly basic oxide among the options, mixture B produces the highest pH solution.
Topic: 11.1
What happens when a piece of magnesium ribbon is placed in cold water?
▶️ Answer/Explanation
Ans: B
Magnesium reacts very slowly with cold water, forming bubbles of hydrogen gas (H₂) on its surface. The reaction is not vigorous (A is incorrect), nor does the magnesium float/react quickly (C is incorrect). The reaction does not produce heat or light (D is incorrect). Thus, the correct observation is B.
Topic: 11.2
The table gives some data for compounds of two elements from Group 2 of the Periodic Table.
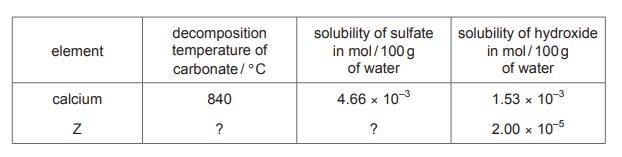
What is the missing data for element Z? 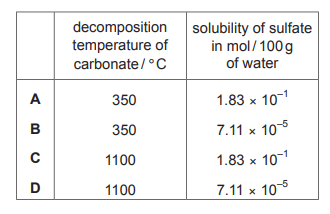
▶️ Answer/Explanation
Ans: A
In Group 2, solubility trends show that sulfates become less soluble down the group, while hydroxides become more soluble. Comparing the given data for element Y (likely magnesium or calcium), element Z must be more soluble in hydroxide but less soluble in sulfate, matching the trend. Thus, the correct missing data is soluble in hydroxide, insoluble in sulfate, corresponding to option A.
Topic: 11.2
Q is a mixture of two compounds of Group 2 elements. Q undergoes thermal decomposition to produce a white solid and only two gaseous products. One of the gaseous products relights a glowing splint. What could be the components of mixture Q?
▶️ Answer/Explanation
Ans: C
1. **Thermal decomposition**: Both Mg(NO₃)₂ and Ca(NO₃)₂ decompose to form white solid oxides (MgO/CaO), NO₂, and O₂.
2. **Gaseous products**: O₂ relights a glowing splint, while NO₂ is the other gas.
3. **Other options**:
– **A**: CaCO₃ decomposes to CO₂ (no O₂).
– **B**: MgCO₃ decomposes to CO₂ (no O₂).
– **D**: MgO and CaO are stable oxides (no decomposition).
Thus, only **Option C** fits all given conditions.
Topic: 9.2
Iodine has a higher melting point than chlorine. What is the reason for this?
▶️ Answer/Explanation
Ans: D
Iodine (\( I_2 \)) has a higher melting point than chlorine (\( Cl_2 \)) because iodine molecules are larger and have more electrons. This results in stronger instantaneous dipole–induced dipole forces (London dispersion forces) between iodine molecules compared to chlorine. These stronger intermolecular forces require more energy to overcome, leading to a higher melting point for iodine.
Topic: 12.1
When concentrated sulfuric acid is added to solid sodium chloride, HCl is formed but not \(Cl_2\). When concentrated sulfuric acid is added to solid sodium iodide, \(I_2\) is formed. Which statement explains these observations?
▶️ Answer/Explanation
Ans: B
1. **Role of Sulfuric Acid**: Concentrated \(H_2SO_4\) acts as an **oxidizing agent**, not a reducing agent (eliminating options C and D). 2. **Reactivity of Halides**: – With \(NaCl\), \(H_2SO_4\) produces \(HCl\) (no \(Cl_2\)), indicating chloride ions (\(Cl^-\)) resist oxidation. – With \(NaI\), \(H_2SO_4\) oxidizes iodide ions (\(I^-\)) to \(I_2\), proving \(I^-\) is **more easily oxidized** than \(Cl^-\).
Topic: 13.4
NaOH(aq) is added to \(NH_4Cl(aq)\). The mixture is warmed. The gas that is produced turns damp red litmus paper blue. Which row is correct?
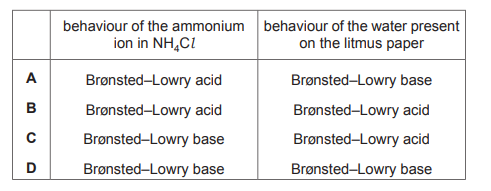
▶️ Answer/Explanation
Ans: B
The reaction is: \[ \text{NaOH} + \text{NH}_4\text{Cl} \rightarrow \text{NaCl} + \text{NH}_3 + \text{H}_2\text{O} \] The gas produced is \(\text{NH}_3\) (ammonia), which is a base and turns damp red litmus paper blue. In the Brønsted–Lowry theory: – \(\text{NH}_4^+\) acts as an acid (donates a proton). – \(\text{NH}_3\) acts as a base (accepts a proton). Thus, the correct row is B (NH3, base, blue).
Topic: 19.2
Artemisinin is a powerful anti-malarial drug.
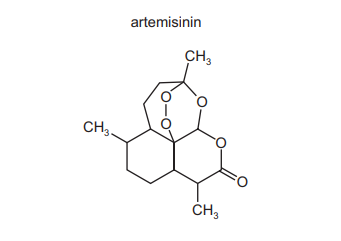
How many chiral centres are there in each molecule of artemisinin?
▶️ Answer/Explanation
Ans: C
A chiral centre is a carbon atom bonded to four different groups. In the structure of artemisinin:
- The 7 carbon atoms marked with asterisks (*) in the structure are chiral centres.
- These include the carbons in the lactone ring and the peroxide bridge that are asymmetrically substituted.
Thus, artemisinin has 7 chiral centres, making option C correct.
Topic: 15.1
Which row shows the correct name and classification of the halogenoalkane shown?
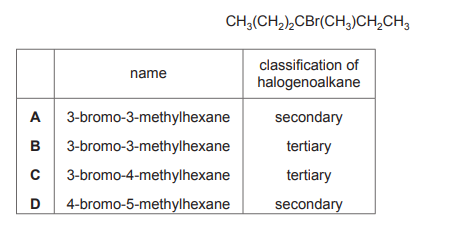
▶️ Answer/Explanation
Ans: B
The structure shows a 5-carbon chain with a bromine atom on the second carbon and a methyl group on the third carbon. – IUPAC Name: 3-methyl-2-bromopentane (methyl is prioritized alphabetically). – Classification: Secondary (2°) halogenoalkane (bromine is attached to a carbon bonded to two other carbons). Thus, Row B is correct.
Topic: 14.1
How many geometrical (cis/trans) isomers are there of hex-2,4-diene, CH₃CH=CHCH=CHCH₃?
▶️ Answer/Explanation
Ans: C
Hex-2,4-diene (CH₃CH=CHCH=CHCH₃) has two double bonds at positions 2 and 4, each capable of exhibiting cis-trans isomerism. The possible combinations are:
- cis-2, cis-4 (both double bonds in cis configuration)
- cis-2, trans-4 (one cis and one trans)
- trans-2, trans-4 (both double bonds in trans configuration)
The fourth possible combination (trans-2, cis-4) is identical to cis-2, trans-4 when rotated, so it does not count as a distinct isomer. Thus, there are 3 distinct geometrical isomers.
Topic: 14.2
The structure of compound X is shown.
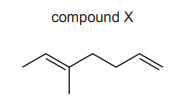
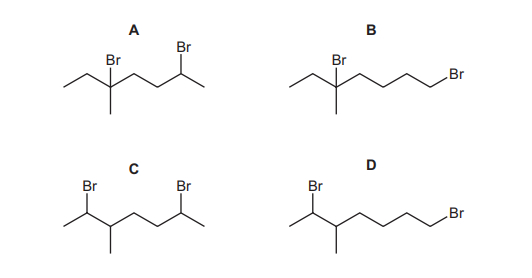
One mole of compound X reacts completely with two moles of hydrogen bromide. What is the structure of the major product of this reaction?
▶️ Answer/Explanation
Ans: A
Compound X is a diene (two double bonds). When it reacts with 2 moles of HBr, the addition follows Markovnikov’s rule, where hydrogen attaches to the carbon with more hydrogens, and bromine attaches to the carbon with fewer hydrogens. The first addition of HBr produces a bromoalkene, and the second addition saturates the remaining double bond, forming a dibromoalkane. The major product is the most stable conformer, where bromines are on adjacent carbons (vicinal dibromide), corresponding to option A.
Topic: 18.1
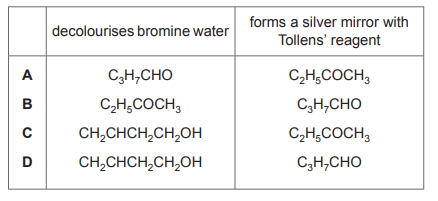
The formulae of three compounds are shown.
\(C_3H_7CHO\) \(C_2H_5COCH_3\) \(CH_2CHCH_2CH_2OH\)
Only one of these compounds will decolourise bromine water. Only one of these compounds will produce a silver mirror with Tollens’ reagent. Which row shows the correct results?
▶️ Answer/Explanation
Ans: D
1. Decolourisation of bromine water: Only \(CH_2CHCH_2CH_2OH\) (but-3-en-1-ol) contains a C=C double bond, which reacts with bromine water (electrophilic addition), causing decolourisation. 2. Silver mirror with Tollens’ reagent: Only \(C_3H_7CHO\) (butanal) is an aldehyde, which is oxidized by Tollens’ reagent to form a silver mirror. Ketones (\(C_2H_5COCH_3\)) and alcohols (\(CH_2CHCH_2CH_2OH\)) do not react with Tollens’ reagent. Thus, Row D correctly identifies these reactions.
Topic: 16.1
Which list contains a compound that is not made during the free radical substitution of methane with chlorine?
▶️ Answer/Explanation
Ans: D
In free radical substitution of methane (CH₄) with chlorine (Cl₂), the expected products are CH₃Cl, CH₂Cl₂, CHCl₃, CCl₄, and small amounts of C₂H₆ (ethane, from radical coupling). C₂H₂Cl₂ (dichloroethene) is not formed in this reaction, as it requires double bonds (not present in methane). Thus, option D contains an invalid product.
Topic: 17.1
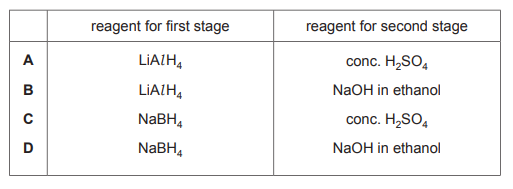
Propanoic acid can be used to make propene by a two-stage synthesis. Which row shows suitable reagents for this synthesis?
▶️ Answer/Explanation
Ans: A
The two-stage synthesis involves: 1. Reduction of propanoic acid to propanol using LiAlH4 (a strong reducing agent). 2. Dehydration of propanol to propene using conc. H2SO4 (eliminates H2O). Only row A correctly lists these reagents in the proper order, making it the correct choice.
Topic: 18.2
Which alcohol reacts with alkaline I₂ (aq) to produce ethanoate ions?
▶️ Answer/Explanation
Ans: C
The reaction described is the iodoform test, which oxidizes a methyl ketone (or a secondary alcohol that can be oxidized to a methyl ketone) to a carboxylate ion (here, ethanoate) and yellow iodoform (CHI₃).
Propan-2-ol (C) is oxidized to propanone (a methyl ketone), which then reacts with alkaline I₂ to form ethanoate ions. The other alcohols either cannot form methyl ketones (A, B) or form longer carboxylate chains (D).
Topic: 18.1
Heating compound X, \(C_7H_{14}O_2\), under reflux with an excess of acidified potassium dichromate(VI) produces compound Y. Compound Y produces hydrogen gas with sodium metal and forms orange crystals with 2,4-DNPH reagent. What could X be?
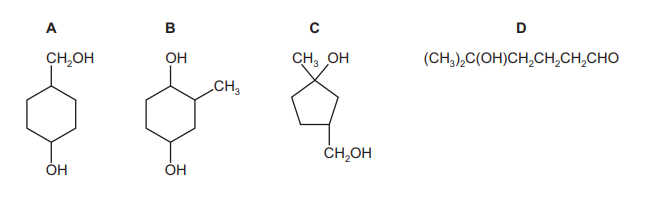
▶️ Answer/Explanation
Ans: A
Key Observations:
1. Oxidation with \( K_2Cr_2O_7 \): Compound X (\( C_7H_{14}O_2 \)) is oxidized to Y, indicating X is likely a secondary alcohol (which oxidizes to a ketone).
2. Reactions of Y:
– Produces \( H_2 \) with Na → Y has an –OH group (carboxylic acid or alcohol).
– Forms orange crystals with 2,4-DNPH → Y is a carbonyl compound (aldehyde/ketone).
Conclusion: Y must be a ketone with an –OH group (a hydroxyketone). Only option A (a secondary alcohol) can oxidize to such a structure.
Topic: 13.3
Which reaction takes place by a nucleophilic addition mechanism?
▶️ Answer/Explanation
Ans: C
1. **Nucleophilic Addition Mechanism**: This occurs when a nucleophile attacks an electrophilic carbon (typically in a carbonyl group, C=O), forming a new bond. 2. **Analysis of Options**: – **A**: Electrophilic addition (propene + HBr). – **B**: Nucleophilic substitution (2-bromopropane + NaOH). – **C**: Nucleophilic addition (propanone’s C=O is attacked by CN⁻ from HCN). – **D**: Free-radical substitution (methane + Cl₂).
Topic: 17.1
Three equations are shown.
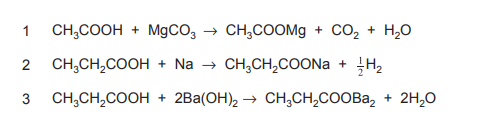
Which of the equations are correct?
▶️ Answer/Explanation
Ans: C
Equation 1: Incorrect. Alkynes (e.g., \(C_2H_2\)) react with \(Br_2\) to form dibromoalkanes, not alkenes. The product should be \(C_2H_2Br_2\). Equation 2: Correct. \(C_2H_4\) (ethene) reacts with steam (\(H_2O\)) in the presence of an acid catalyst to form ethanol (\(C_2H_5OH\)). Equation 3: Incorrect. \(C_2H_5OH\) undergoes elimination (not substitution) with concentrated \(H_2SO_4\) to form \(C_2H_4\), not \(C_2H_6\). Thus, only Equation 2 is correct.
Topic: 19.1
How many esters with the molecular formula \(C_5H_{10}O_2\) can be made by reacting a primary alcohol with a carboxylic acid?
▶️ Answer/Explanation
Ans: C
To determine the number of esters with the formula \(C_5H_{10}O_2\) formed from a primary alcohol and carboxylic acid:
- Identify possible alcohol-acid pairs: The ester has the general formula \(RCOOR’\), where \(R + R’ = C_4H_9\) (since \(C_5H_{10}O_2 – COO = C_4H_9\)).
- Primary alcohol combinations:
- Methanol (\(CH_3OH\)) + Butanoic acid (\(C_3H_7COOH\)) → 2 esters (straight-chain and branched).
- Ethanol (\(C_2H_5OH\)) + Propanoic acid (\(C_2H_5COOH\)) → 2 esters.
- Propan-1-ol (\(C_3H_7OH\)) + Ethanoic acid (\(CH_3COOH\)) → 2 esters.
- Total esters: 2 (from methanol) + 2 (from ethanol) + 2 (from propanol) = 6 esters.
Thus, the correct answer is C (6).
Topic: 19.1
The diagram shows an ester. It is heated under reflux with an excess of NaOH(aq).
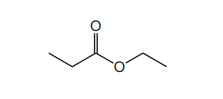
Which row shows the 2 products of the reaction?
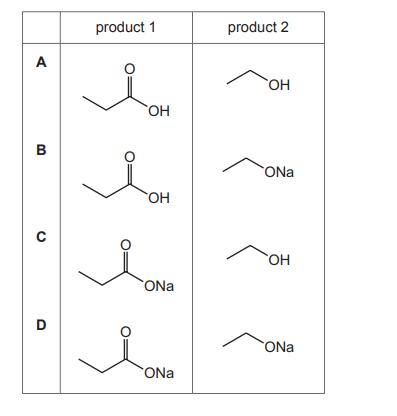
▶️ Answer/Explanation
Ans: C
The ester shown is ethyl ethanoate (CH3COOCH2CH3). When heated with NaOH(aq), it undergoes alkaline hydrolysis (saponification), producing: \[ \text{CH}_3\text{COOCH}_2\text{CH}_3 + \text{NaOH} \rightarrow \text{CH}_3\text{COO}^-\text{Na}^+ + \text{CH}_3\text{CH}_2\text{OH} \] – Product 1: Sodium ethanoate (CH3COO–Na+) – Product 2: Ethanol (CH3CH2OH) Thus, Row C is correct.
Topic: 1.1
Oxygen has three stable isotopes, \(^{16}O\), \(^{17}O\) and \(^{18}O\). All three isotopes are present in a sample of oxygen gas, O₂, which was analysed using a mass spectrometer. How many peaks associated with the \(O_2^+\) ion would be expected?
▶️ Answer/Explanation
Ans: B
The \(O_2^+\) ion can form from all possible combinations of the three oxygen isotopes (\(^{16}O\), \(^{17}O\), \(^{18}O\)). The number of distinct peaks corresponds to the unique mass combinations:
- \(^{16}O\)-\(^{16}O\) → Mass = 32
- \(^{16}O\)-\(^{17}O\) → Mass = 33
- \(^{16}O\)-\(^{18}O\) → Mass = 34
- \(^{17}O\)-\(^{17}O\) → Mass = 34 (distinct from \(^{16}O\)-\(^{18}O\) due to different isotopic composition)
- \(^{17}O\)-\(^{18}O\) → Mass = 35
- \(^{18}O\)-\(^{18}O\) → Mass = 36
However, \(^{16}O\)-\(^{18}O\) and \(^{17}O\)-\(^{17}O\) both have the same mass (34), but they are distinct species. The mass spectrometer cannot distinguish between them based on mass alone, but they are chemically distinct. Typically, the question considers unique mass peaks, leading to 5 distinct peaks (32, 33, 34, 35, 36).
