Question
(a) Table 2.1 shows eight ions that are biologically important.
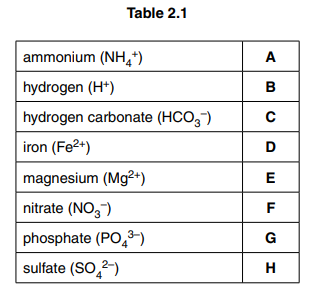
Choose one ion to match each of the following statements. In each case write one letter from Table 2.1. You may use each letter (A to H) once, more than once or not at all.
(i) A component of polynucleotides.[1]
(ii) Ion produced by enzyme activity inside red blood cells.[1]
(iii) Ion used in the production of all amino acids in chloroplasts.[1]
(iv) Ion that diffuses through carrier proteins with sucrose into companion cells in phloem tissue.[1]
(v) Component of haem group in haemoglobin that binds oxygen.[1]
(b) The enzyme nitrogenase is found in free-living and symbiotic nitrogen-fixing bacteria. Nitrogenase catalyses the reaction:
\(N_{2\left ( g \right )}+ 6 e^{-} + 8H^{+}_{\left ( aq \right )}\rightarrow 2NH{_{4}}^{+} {_{\left ( aq \right )}}\)
Some nitrogenase enzymes have vanadium ions in their active sites; others have molybdenum ions.
Explain how the enzyme nitrogenase functions in the fixation of nitrogen.[4]
(c) Some pea plants were grown with their roots in a solution of mineral ions. The solution was kept aerated for three days.
The concentrations of five ions in the solution and in the root tissue were determined after the three days. The results are shown in Table 2.2.
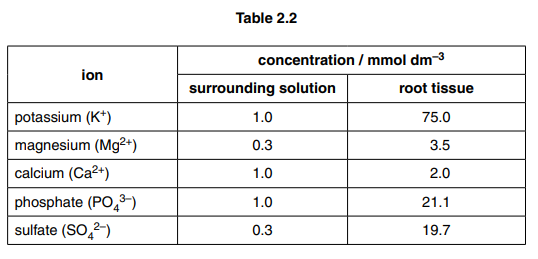
With reference to Table 2.2, suggest how cell surface membranes of root cells are responsible for the concentrations of ions in the roots compared to the surrounding solution.[5] [Total: 14]
Answer/Explanation
Ans:
2 (a) (i) G ;
(ii) B/C ;
(iii) A/F ;
(iv) B ;
(v) D ;
(b) 1 nitrogen and hydrogen/ substrates, bind to/AW, active site ;
2 enzyme-substrate complex (forms) ;
3 ref. lock and key / induced fit, mechanism ;
4 activation energy of reaction is lowered ;
5 example of how activation energy lowered ;
e.g. strain on (triple) bond of, N2 / (di)nitrogen
A bond broken between nitrogen (atoms)
nitrogen and hydrogen ions held close together for bond formation
transfer of electrons
alternative pathway
6 product/NH4+, leaves active site ;
7 ATP, required/ used/provided from respiration ;
8 ref. anaerobic conditions for enzyme action ;
9 suggestion as to use of, vanadium/ molybdenum, in active site ;
e.g. act as cofactor/ coenzyme
transfer of, electrons /protons
(c) 1 concentration of all the ions is greater in the root tissue than in the solution ; ora A roots
2 comparative data quote ;
according to these data
3 (so) ions will not diffuse into the root tissue ;
A if (facilitated) diffusion only, initially / till equilibrium reached
4 (so) active transport ; A active, uptake/ pumping I facilitated diffusion
5 use ATP ; A energy
R ATP energy
6 move ions, against concentration gradient/ from low to high concentration;
A diffusion gradient
7 use, membrane/ integral/ intrinsic / transmembrane/ transport/ carrier, proteins ;
R channel proteins
8 are specific / have specific binding sites ;
9 involve, conformational/ shape, change ;
10 comparative data quote to suggest that some ions are pumped more than others ;
e.g. steepest gradients for K+ and SO 4–
11 phospholipid bilayer/ hydrophobic core (of cell surface membrane) is impermeable to ions ;
12 so ions cannot diffuse out/(membrane) proteins only allow inward flow of ions ;
13 AVP ; e.g. suggestion of differing numbers of specific membrane proteins to explain observation of mp 10
Question
Statements A to E relate to biological molecules.
For each statement, identify the most appropriate term that matches the description.
A The molecule formed from a condensation reaction between fructose and glucose.
B The name of the bond broken when two amino acids are separated by hydrolysis
C The unbranched polymer consisting only of β-glucose molecules.
D The reagent used to test for the presence of proteins.
E The molecule produced, in addition to fatty acids, when a triglyceride is hydrolysed.[5] [Total: 5]
Answer/Explanation
Ans:
1
A sucrose ;
B peptide ; A amide
C cellulose ;
D biuret ; A (dilute) potassium/ sodium, hydroxide (solution) and (dilute) copper sulfate (solution)
R Millon’s solution
E glycerol ;
Question
(a) A student investigated the effect of changing the surface area to volume ratio on diffusion.
- Two different-sized blocks of agar, X and Y, were made.
- The agar contained Universal Indicator solution.
- Universal Indicator solution changes colour when acid is added.
- The blocks were placed in dilute hydrochloric acid at the same temperature.
- The student timed how long it took for each block to change colour completely.
Blocks X and Y are shown in Fig. 6.1. All dimensions are in cm.
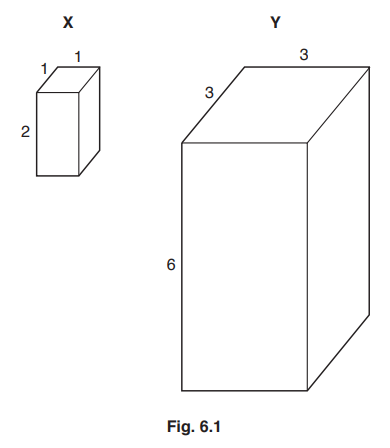
(i) The surface area to volume ratio of block X is 5:1.
Calculate the surface area to volume ratio of block Y.
Show your working.[2]
(ii) The student observed that block X changed colour completely in a much shorter time than block Y.
Explain why.[2]
(iii) Suggest how the results of this investigation help to explain why plants need a transport system.[2]
(b) An experiment was carried out by the student to investigate the ability of reducing sugars to diffuse through Visking tubing. Fig. 6.2 shows the apparatus used.
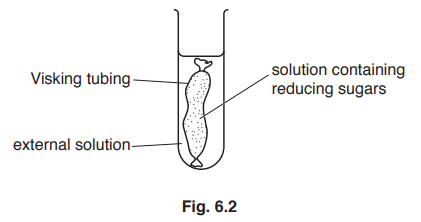
At the start of the experiment, the external solution did not contain any reducing sugars.
At intervals, the student tested for the presence of reducing sugars, both within the Visking tubing and in the external solution.
Name the reagent that is used to test for the presence of reducing sugars.[1] [Total: 7]
Answer/Explanation
Ans:
6(a)(i) surface area : volume = 1.67 : 1 ; ; A 1.7 : 1, 5 : 3
if incorrect, allow one mark for working
surface area = 90 mm2 and volume = 54 mm3
calculations:
surface area volume ratio
6 × 3 × 4 (sides) = 72 mm2 6 × 3 × 3 90 : 54
3 × 3 × 2 (sides) = 18 mm2
6(a)(ii) (block X) has higher, surface area to volume ratio /SA:V ;
OR
(block X) has more surface area proportionately per unit volume /AW ;
reference to shorter distance for diffusion to centre ;
6(a)(iii) two from:
1 diffusion (rate) too slow ; A idea of cannot rely on diffusion
2 reference to distances too far to reach all, cells /tissues ;
3 time taken is too long/AW ;
6(b) Benedict’s (reagent / solution) ;
