Which description identifies a reversible, non-competitive enzyme inhibitor?
▶️ Answer/Explanation
Ans: B
A non-competitive inhibitor binds to an allosteric site (not the active site), changing the enzyme’s shape reversibly. Option A describes competitive inhibition, C describes mixed inhibition, and D describes uncompetitive inhibition. Only B correctly describes non-competitive inhibition.
A student investigated the effect of substrate concentration on the rate of an enzyme-catalysed reaction.
The student plotted the results in a graph.
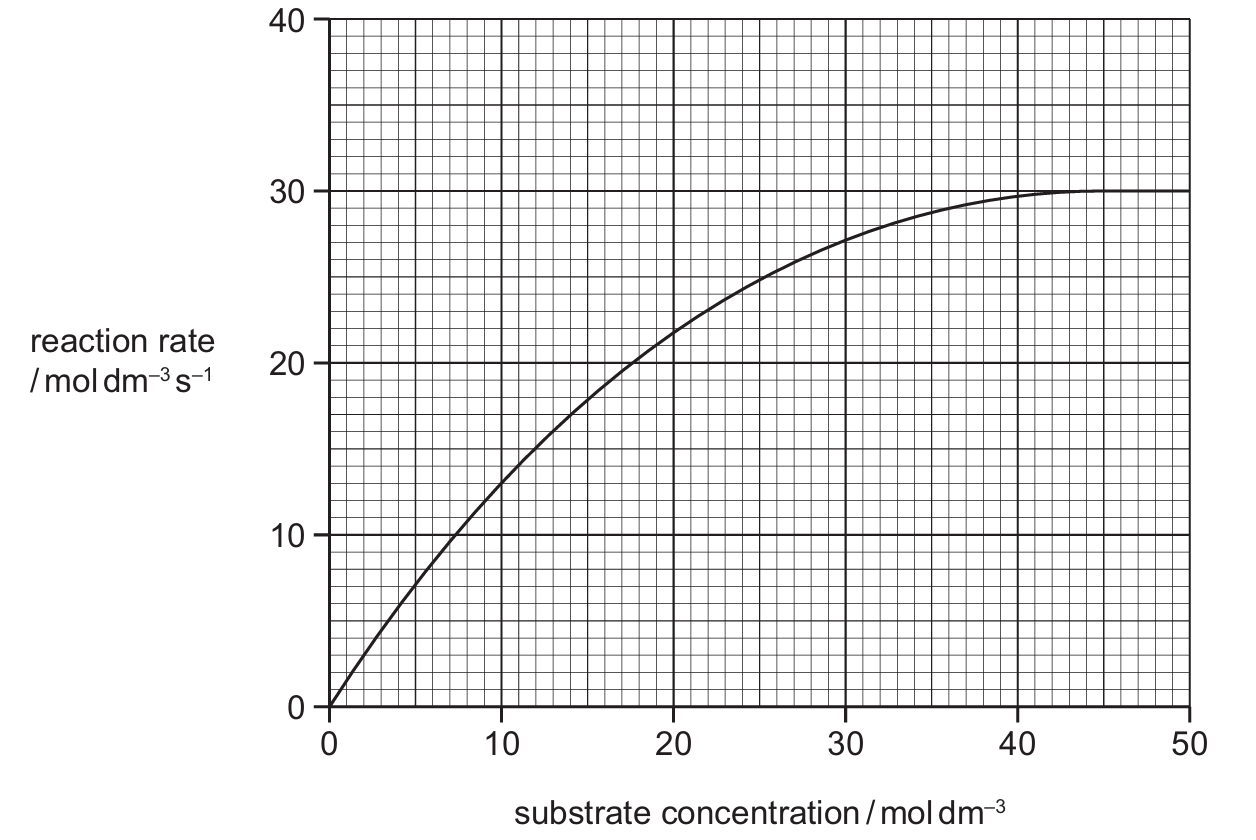
What is the Km for this enzyme-catalysed reaction?
▶️ Answer/Explanation
Ans: A
Km (Michaelis constant) is the substrate concentration at half the maximum reaction rate. From the graph, when rate is 20 (half of Vmax = 40), the corresponding substrate concentration is 12 mol dm-3. Options B and C are incorrect as Km is measured in concentration units, not rate units.
Two different enzymes, P and Q, are investigated to find the optimum pH for each enzyme. The results show that P works only in acidic conditions. Q has an optimum pH which is slightly alkaline.
Which graph shows the correct results for P and Q?
▶️ Answer/Explanation
Ans: B
Graph B correctly shows P active only in acidic pH (left of 7) and Q with peak activity in alkaline pH (right of 7). Others show wrong pH ranges (A/C) or missing pH scale (D).
HIV-1 protease is an enzyme produced by the HIV virus.
Two identical chains of 99 amino acids form the enzyme. In each chain, amino acids 25, 26 and 27 in the sequence form part of the active site.
Which orders of protein structure control the shape of the active site?
▶️ Answer/Explanation
Ans: A
The active site shape depends on all structural levels: primary (sequence determines position), secondary/tertiary (folding creates 3D shape), and quaternary (interaction of two chains forms complete active site).
