CIE AS/A Level Biology -12.2 Respiration- Study Notes- New Syllabus
CIE AS/A Level Biology -12.2 Respiration- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -12.2 Respiration- Study Notes- New Syllabus
Key Concepts:
- State where each of the four stages in aerobic respiration occurs in eukaryotic cells:
• glycolysis in the cytoplasm
• link reaction in the mitochondrial matrix
• Krebs cycle in the mitochondrial matrix
• oxidative phosphorylation on the inner membrane of mitochondria - outline glycolysis as phosphorylation of glucose and the subsequent splitting of fructose 1,6-bisphosphate (6C) into two triose phosphate molecules (3C), which are then further oxidised to pyruvate (3C), with the production of ATP and reduced NAD
- explain that, when oxygen is available, pyruvate enters mitochondria to take part in the link reaction
- describe the link reaction, including the role of coenzyme A in the transfer of acetyl (2C) groups
- outline the Krebs cycle, explaining that oxaloacetate (4C) acts as an acceptor of the 2C fragment from acetyl coenzyme A to form citrate (6C), which is converted back to oxaloacetate in a series of small steps
- explain that reactions in the Krebs cycle involve decarboxylation and dehydrogenation and the reduction of the coenzymes NAD and FAD
- describe the role of NAD and FAD in transferring hydrogen to carriers in the inner mitochondrial membrane
- explain that during oxidative phosphorylation:
• hydrogen atoms split into protons and energetic electrons
• energetic electrons release energy as they pass through the electron transport chain (details of carriers are not expected)
• the released energy is used to transfer protons across the inner mitochondrial membrane
• protons return to the mitochondrial matrix by facilitated diffusion through ATP synthase, providing energy for ATP synthesis (details of ATP synthase are not expected)
• oxygen acts as the final electron acceptor to form water - describe the relationship between the structure and function of mitochondria using diagrams and electron micrographs
- outline respiration in anaerobic conditions in mammals (lactate fermentation) and in yeast cells (ethanol fermentation)
- explain why the energy yield from respiration in aerobic conditions is much greater than the energy yield from respiration in anaerobic conditions (a detailed account of the total yield of ATP from the aerobic respiration of glucose is not expected)
- explain how rice is adapted to grow with its roots submerged in water, limited to the development of aerenchyma in roots, ethanol fermentation in roots and faster growth of stems
- describe and carry out investigations using redox indicators, including DCPIP and methylene blue, to determine the effects of temperature and substrate concentration on the rate of respiration of yeast
- describe and carry out investigations using simple respirometers to determine the effect of temperature on the rate of respiration
Aerobic Respiration: Sites of the 4 Stages
📌 Overview
- Aerobic respiration is the process by which cells release energy (ATP) from glucose in the presence of oxygen.
- It occurs in a sequence of four main stages, each located in a specific part of the eukaryotic cell.
1. Glycolysis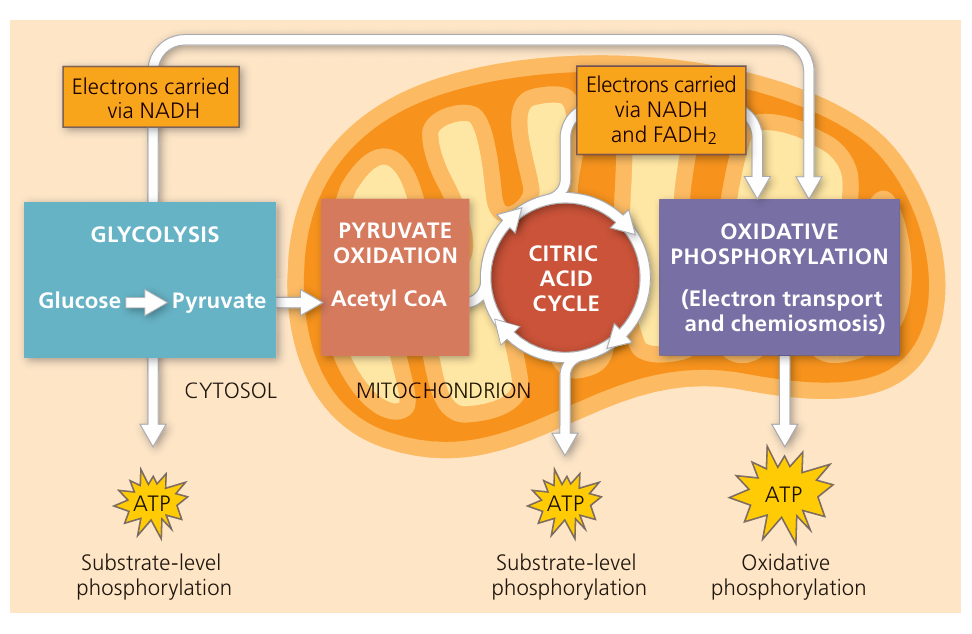
- Location: Cytoplasm
- What happens:
- Glucose (6C) is broken down into two molecules of pyruvate (3C).
- Small yield of ATP (substrate-level phosphorylation).
- Production of reduced NAD (NADH).
2. Link Reaction
- Location: Mitochondrial matrix
- What happens:
- Pyruvate (3C) is decarboxylated and dehydrogenated.
- Forms acetyl-CoA (2C).
- Releases CO₂ and reduces NAD⁺ → NADH.
3. Krebs Cycle (Citric Acid Cycle)
- Location: Mitochondrial matrix
- What happens:
- Acetyl-CoA (2C) combines with oxaloacetate (4C) to form citrate (6C).
- Series of reactions release CO₂, ATP, NADH, and FADH₂.
- Cycle regenerates oxaloacetate.
4. Oxidative Phosphorylation (Electron Transport Chain + Chemiosmosis)
- Location: Inner mitochondrial membrane (cristae)
- What happens:
- NADH and FADH₂ donate electrons to the electron transport chain.
- Energy released pumps protons (H⁺) into intermembrane space.
- Protons flow back via ATP synthase → large amounts of ATP produced.
- Oxygen is the final electron acceptor, combining with H⁺ and electrons to form water.
📊 Table Summary
| Stage | Location | Main Outputs |
|---|---|---|
| Glycolysis | Cytoplasm | 2 Pyruvate, 2 ATP, 2 NADH |
| Link Reaction | Mitochondrial matrix | Acetyl-CoA, CO₂, NADH |
| Krebs Cycle | Mitochondrial matrix | CO₂, ATP, NADH, FADH₂ |
| Oxidative Phosphorylation | Inner mitochondrial membrane | Large ATP, H₂O |
– Cytoplasm = glycolysis
– Mitochondrial matrix = link reaction & Krebs cycle
– Inner mitochondrial membrane = oxidative phosphorylation
Glycolysis
📌 Overview
- Glycolysis is the first stage of aerobic (and anaerobic) respiration, occurring in the cytoplasm.
- It breaks down glucose (6C) into pyruvate (3C) with a small yield of ATP and reduced NAD.
🔬 Stages of Glycolysis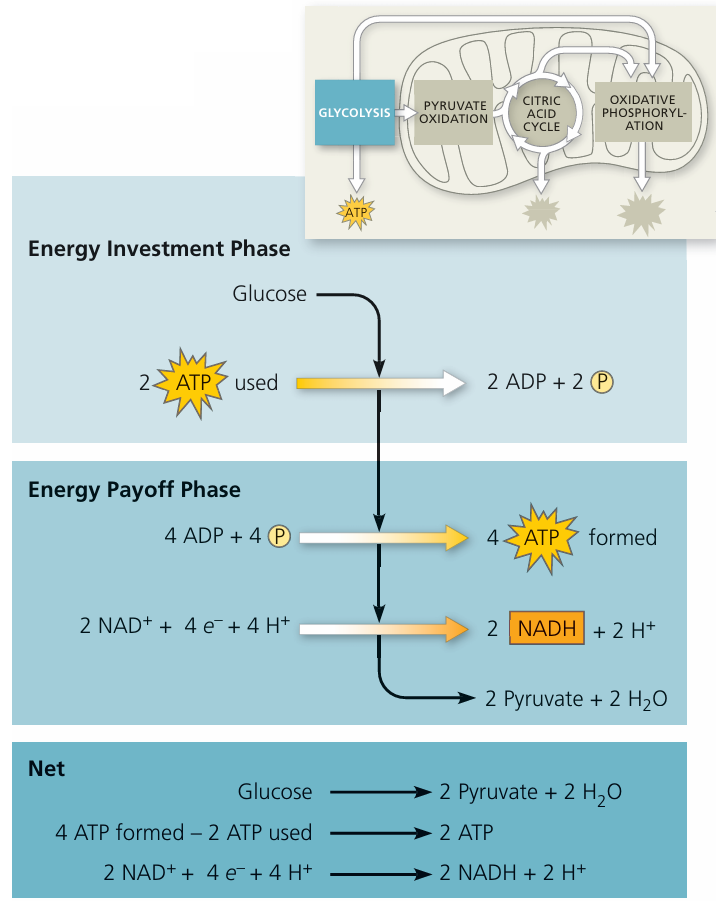
1. Phosphorylation of Glucose
- Glucose (6C) is phosphorylated using 2 ATP molecules.
- Forms fructose 1,6-bisphosphate (6C), which is more reactive.
2. Splitting (Lysis) of Fructose 1,6-bisphosphate
- The 6C sugar splits into two triose phosphate (TP) molecules (3C each).
3. Oxidation of Triose Phosphate
- Each TP is oxidised:
- Hydrogen atoms are removed and accepted by NAD⁺ → reduced NAD (NADH).
- Energy released phosphorylates ADP → ATP.
4. Formation of Pyruvate
- Each triose phosphate (3C) is converted into pyruvate (3C).
⚡ Net Yield of Glycolysis (per glucose)
- ATP: 4 produced – 2 used = net 2 ATP
- NADH: 2 reduced NAD
- Pyruvate: 2 pyruvate molecules (enter mitochondria if O₂ present, or undergo fermentation if O₂ absent)
📊 Summary Table
| Step | Process | Key Outputs |
|---|---|---|
| 1. Phosphorylation | Glucose → fructose 1,6-bisphosphate | Uses 2 ATP |
| 2. Splitting (lysis) | 6C sugar → two 3C triose phosphates | 2 TP formed |
| 3. Oxidation of TP | TP oxidised, NAD⁺ reduced | 2 NADH, 2 ATP produced |
| 4. Pyruvate formation | TP → pyruvate | 2 pyruvate, 2 ATP formed |
– Glycolysis = glucose (6C) → 2 pyruvate (3C)
– Produces: net 2 ATP + 2 NADH
– Happens in the cytoplasm
– Does not require oxygen (anaerobic stage).
Pyruvate Entry into Mitochondria and the Link Reaction
📌 Overview
- At the end of glycolysis, 2 pyruvate molecules (3C each) are formed in the cytoplasm.
- When oxygen is available (aerobic conditions), pyruvate is actively transported into the mitochondrial matrix.
- This enables the link reaction, which connects glycolysis to the Krebs cycle for further energy production.
🌱 Steps of the Link Reaction
1. Decarboxylation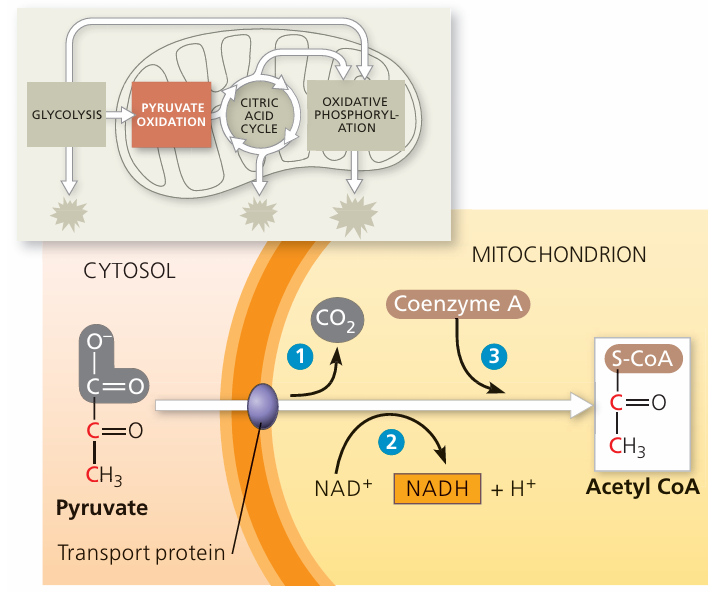
- Pyruvate (3C) loses one carbon as CO₂.
- Forms acetate (2C).
2. Oxidation
- Hydrogen atoms are removed from acetate.
- Accepted by NAD⁺ → reduced NAD (NADH).
3. Formation of Acetyl Coenzyme A
- Acetate (2C) combines with coenzyme A (CoA).
- Forms Acetyl CoA (2C), which enters the Krebs cycle.
⚡ Role of Oxygen
- Oxygen is required for aerobic respiration.
- Ensures that NADH produced in glycolysis and the link reaction can later donate electrons to the electron transport chain.
- Without oxygen, pyruvate cannot enter mitochondria and is instead converted to lactate or ethanol (fermentation).
📊 Products per Pyruvate
| Product | Quantity per Pyruvate | Function/Notes |
|---|---|---|
| CO₂ | 1 | Waste gas released |
| NADH | 1 | Carries electrons to ETC |
| Acetyl CoA | 1 | Enters Krebs cycle for further ATP |
Per glucose (2 pyruvates): 2 CO₂, 2 NADH, 2 Acetyl CoA
– Pyruvate must enter mitochondria for aerobic respiration.
– Link reaction occurs in the mitochondrial matrix.
– Produces CO₂, NADH, and acetyl CoA.
– Oxygen presence is essential to allow continuation of aerobic respiration.
Link Reaction (Acetyl CoA Formation)
📌 Purpose
- Connects glycolysis to the Krebs cycle.
- Converts pyruvate (3C) → acetyl CoA (2C) for aerobic respiration.
- Occurs in the mitochondrial matrix.
🌱 Steps
1. Decarboxylation
- Removes one carbon as CO₂ from pyruvate.
2. Oxidation
- Hydrogen atoms are removed from acetate.
- NAD⁺ is reduced → NADH.
3. Acetyl Group Transfer
- Acetate (2C) combines with coenzyme A (CoA) → acetyl CoA.
- CoA safely transports the acetyl group to the Krebs cycle.
⚡ Products (per pyruvate)
| Product | Quantity | Notes |
|---|---|---|
| CO₂ | 1 | Waste gas |
| NADH | 1 | Electron carrier |
| Acetyl CoA | 1 | Enters Krebs cycle |
Per glucose (2 pyruvate): 2 CO₂, 2 NADH, 2 Acetyl CoA
– Link reaction is mitochondrial and aerobic.
– Produces acetyl CoA for the Krebs cycle.
– Coenzyme A is essential for transferring acetyl groups safely.
Krebs Cycle (Citric Acid Cycle)
📌 Overview
- Takes place in the mitochondrial matrix.
- Purpose: release energy from acetyl CoA in a controlled series of reactions.
- Cyclic pathway: Acetyl (2C) enters → citrate (6C) formed → series of reactions → oxaloacetate (4C) regenerated.
🌱 Key Steps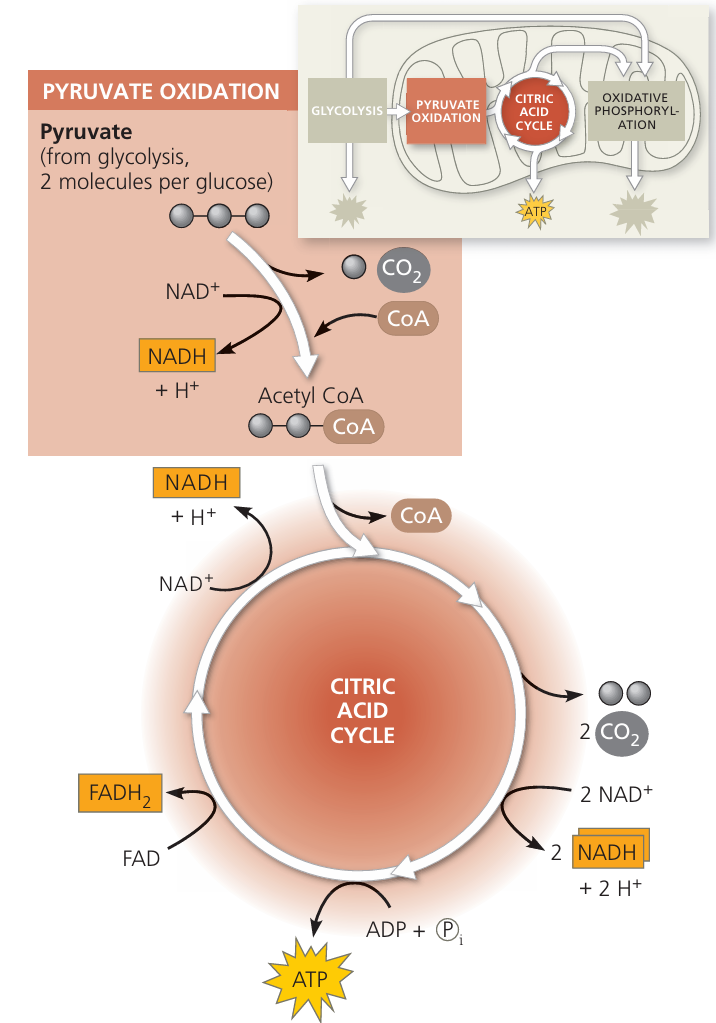
1. Formation of Citrate
- Acetyl CoA (2C) combines with oxaloacetate (4C) → citrate (6C).
- Coenzyme A is released to be reused.
2. Series of Small Steps
- Citrate undergoes rearrangements and decarboxylation.
- Two carbons released as CO₂.
- Hydrogen atoms are removed → NAD⁺ → NADH and FAD → FADH₂.
- ATP (or GTP) is produced via substrate-level phosphorylation.
3. Regeneration of Oxaloacetate
- After all steps, oxaloacetate (4C) is regenerated.
- Cycle is ready to accept another acetyl CoA molecule.
⚡ Net Products (per acetyl CoA)
| Product | Quantity | Notes |
|---|---|---|
| CO₂ | 2 | Waste gas |
| NADH | 3 | Electron carrier |
| FADH₂ | 1 | Electron carrier |
| ATP (GTP) | 1 | Direct energy for cellular work |
Per glucose (2 acetyl CoA): 4 CO₂, 6 NADH, 2 FADH₂, 2 ATP
– Oxaloacetate accepts the 2C acetyl group to form citrate.
– Citrate is converted back to oxaloacetate through a series of reactions.
– Produces CO₂, reduced NAD/FAD, and ATP.
– Provides electrons for oxidative phosphorylation to generate the bulk of ATP.
Reactions in the Krebs Cycle
📌 Key Reaction Types
1. Decarboxylation
- Removal of carbon atoms from intermediates as CO₂.
- Reduces carbon chain length (e.g., citrate 6C → 5C → 4C).
- CO₂ is waste gas, released from the cell.
2. Dehydrogenation (Oxidation)
- Hydrogen atoms are removed from intermediates.
- Hydrogen is accepted by coenzymes:
- NAD⁺ → NADH
- FAD → FADH₂
- These reduced coenzymes carry high-energy electrons to the electron transport chain.
3. Substrate-Level Phosphorylation
- Small amount of ATP (or GTP) produced directly.
⚡ Summary of Coenzyme Reduction
| Step | Coenzyme Reduced | Notes |
|---|---|---|
| Isocitrate → α-Ketoglutarate | NAD⁺ → NADH | 1st dehydrogenation, 1st CO₂ released |
| α-Ketoglutarate → Succinyl CoA | NAD⁺ → NADH | 2nd dehydrogenation, 2nd CO₂ released |
| Succinate → Fumarate | FAD → FADH₂ | Transfers electrons to ETC |
| Malate → Oxaloacetate | NAD⁺ → NADH | Final dehydrogenation before cycle restarts |
– Decarboxylation removes CO₂.
– Dehydrogenation transfers electrons to NAD⁺ and FAD, forming NADH and FADH₂.
– Reduced coenzymes are essential for oxidative phosphorylation, where most ATP is generated.
– The cycle regenerates oxaloacetate to accept the next acetyl group.
Role of NAD and FAD in Aerobic Respiration
📌 Overview
- NAD (Nicotinamide adenine dinucleotide) and FAD (Flavin adenine dinucleotide) are coenzymes.
- They collect hydrogen atoms (electrons + protons) during glycolysis, link reaction, and Krebs cycle.
- They transfer these high-energy electrons to the electron transport chain (ETC) in the inner mitochondrial membrane.
🌱 How They Work
1. Hydrogen Collection
- During oxidation reactions:
- NAD⁺ + 2H → NADH + H⁺
- FAD + 2H → FADH₂
2. Electron Transfer
- NADH and FADH₂ donate electrons to electron carriers in the inner mitochondrial membrane.
- Electrons flow through the ETC, releasing energy to pump H⁺ into the intermembrane space.
3. Proton Gradient Formation
- H⁺ accumulate in the intermembrane space → creates proton motive force.
- Protons flow back through ATP synthase → drives ATP production.
⚡ Key Differences Between NAD and FAD
| Coenzyme | Entry Point in ETC | ATP Yield per Molecule |
|---|---|---|
| NADH | Complex I (early in chain) | ~3 ATP |
| FADH₂ | Complex II (later in chain) | ~2 ATP |
– NAD and FAD are electron carriers, transporting H from metabolic reactions to the ETC.
– Their reduced forms (NADH, FADH₂) are essential for oxidative phosphorylation.
– This process is how the energy from glucose is converted into ATP.
Oxidative Phosphorylation
📌 Overview
- Takes place on the inner mitochondrial membrane.
- Uses electrons from NADH and FADH₂ to produce the majority of ATP.
- Oxygen is essential as the final electron acceptor.
🌱 Key Steps
1. Splitting of Hydrogen
- NADH and FADH₂ release hydrogen atoms.
- H → protons (H⁺) + energetic electrons (e⁻).
2. Electron Transport Chain (ETC)
- Electrons flow through the ETC, releasing energy gradually.
- Energy is used to pump protons (H⁺) across the inner mitochondrial membrane → creates proton gradient.
3. Proton Return & ATP Synthesis
- Protons move back into the mitochondrial matrix via facilitated diffusion through ATP synthase.
- Flow of protons drives ATP synthesis from ADP + Pi.
4. Formation of Water
- Electrons combine with oxygen (O₂) and protons → form water (H₂O).
- Oxygen is the final electron acceptor, preventing electron backup.
– Oxidative phosphorylation is aerobic.
– Produces the majority of ATP in aerobic respiration (~28–34 ATP per glucose).
– Depends on proton gradient and oxygen to drive ATP production.
– NADH and FADH₂ supply the electrons; oxygen removes them at the end.
Structure and Function of Mitochondria
📌 Overview
- Mitochondria are the powerhouses of the cell.
- Site of aerobic respiration, where glucose-derived energy is converted into ATP.
- Structure is highly adapted to maximize energy production.
🌱 Key Structural Features
| Structure | Function |
|---|---|
| Double membrane | Outer membrane encloses the organelle; inner membrane is folded into cristae to increase surface area for oxidative phosphorylation. |
| Cristae | Provides large surface area for electron transport chain and ATP synthase. |
| Matrix | Contains enzymes for Krebs cycle and mitochondrial DNA + ribosomes for protein synthesis. |
| Intermembrane space | Site of proton accumulation, creating a proton gradient for ATP synthesis. |
| Mitochondrial DNA & ribosomes | Allow mitochondria to produce some proteins and enzymes independently of the nucleus. |
🔬 How Diagrams & Electron Micrographs Help
- Diagram (labelled): Shows cristae, matrix, outer membrane, and intermembrane space clearly for understanding function.
- Electron micrograph: Shows actual internal structure, highlighting cristae density and matrix content.
- Interpretation: High cristae surface area = high ATP production potential; more matrix enzymes = efficient Krebs cycle.
– Double membrane → separates reactions, allows proton gradient formation.
– Cristae → maximize area for electron transport and ATP synthase.
– Matrix enzymes → catalyze Krebs cycle efficiently.
– Mitochondrial DNA → allows rapid synthesis of proteins needed for respiration.
– Overall: Structure is optimized for energy production in aerobic respiration.
Anaerobic Respiration
📌 Overview
- Occurs when oxygen is absent.
- Produces ATP without oxygen, but less efficiently than aerobic respiration.
- Different pathways in mammals and yeast.
🌱 1. Mammals: Lactate Fermentation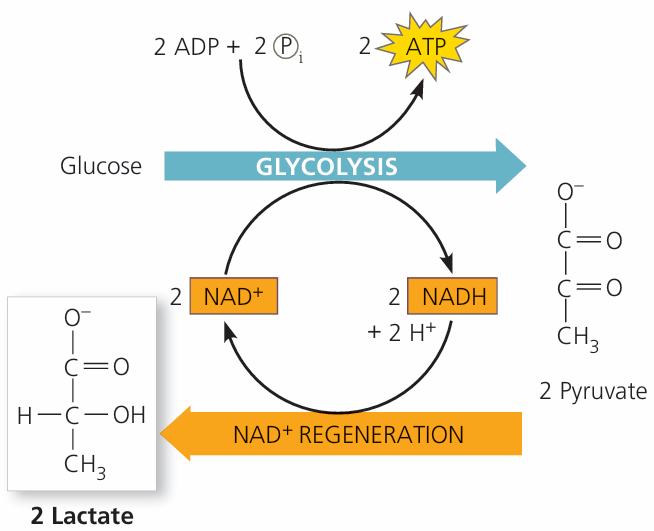
- Glycolysis still occurs → 2 ATP per glucose.
- Pyruvate (3C) is converted to lactate (3C):
Reaction: Pyruvate + NADH → Lactate + NAD⁺ - NAD⁺ is regenerated → allows glycolysis to continue.
- Lactate accumulation can cause muscle fatigue.
- Key points:
- Location: Cytoplasm
- ATP yield: 2 ATP per glucose
- End product: Lactate
- Reversible when oxygen becomes available → lactate converted back to pyruvate in the liver.
🌱 2. Yeast: Ethanol Fermentation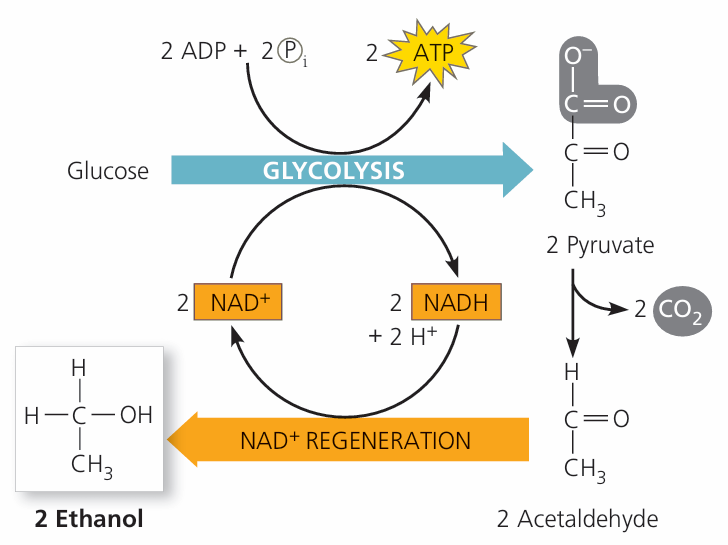
- Glycolysis still occurs → 2 ATP per glucose.
- Pyruvate (3C) is converted to ethanol (2C) and CO₂:
Step 1: Pyruvate → Acetaldehyde + CO₂
Step 2: Acetaldehyde + NADH → Ethanol + NAD⁺ - NAD⁺ is regenerated → allows glycolysis to continue.
- Key points:
- Location: Cytoplasm
- ATP yield: 2 ATP per glucose
- End products: Ethanol + CO₂
- Basis for alcoholic fermentation in brewing and baking.
📊 Comparison Table
| Feature | Mammals (Lactate) | Yeast (Ethanol) |
|---|---|---|
| Location | Cytoplasm | Cytoplasm |
| ATP yield per glucose | 2 | 2 |
| End product | Lactate | Ethanol + CO₂ |
| Purpose | Regenerate NAD⁺ | Regenerate NAD⁺ |
| Reversibility | Lactate → Pyruvate (liver) | Irreversible in cell |
– Anaerobic respiration allows glycolysis to continue in the absence of oxygen.
– Produces small amounts of ATP quickly.
– Mammals produce lactate, yeast produce ethanol and CO₂.
Energy Yield: Aerobic vs Anaerobic Respiration
📌 Key Points
Aerobic Respiration
- Uses oxygen as the final electron acceptor.
- Complete breakdown of glucose to CO₂ and H₂O.
- All carbon atoms are fully oxidized, and high-energy electrons from NADH and FADH₂ are transferred to the electron transport chain.
- Produces much more ATP because oxidative phosphorylation generates the majority of energy.
Anaerobic Respiration
- No oxygen available → no electron transport chain.
- Glucose is only partially broken down (to lactate in mammals, ethanol + CO₂ in yeast).
- Only glycolysis produces ATP → 2 ATP per glucose.
- Electrons from NADH are used to regenerate NAD⁺ but no additional ATP is produced.
🔍 Why Aerobic > Anaerobic
| Factor | Aerobic Respiration | Anaerobic Respiration |
|---|---|---|
| Final electron acceptor | Oxygen (efficient) | Pyruvate / Acetaldehyde |
| Complete oxidation of glucose | Yes | No |
| ATP yield per glucose | High (~30–38 ATP) | Low (2 ATP) |
| Energy released | Most of glucose energy | Only small fraction |
– Aerobic respiration produces much more energy because glucose is fully oxidized and energy from electrons is captured in oxidative phosphorylation.
– Anaerobic respiration is less efficient, yielding only the small amount of energy from glycolysis.
Adaptations of Rice to Waterlogged Conditions
📌 Overview
- Rice is a semi-aquatic plant.
- Grows in paddy fields where roots are submerged.
- Adaptations allow it to survive low oxygen (hypoxic) conditions in waterlogged soil.
🌱 Key Adaptations
Aerenchyma in Roots
- Roots develop air spaces (aerenchyma).
- Allows oxygen to diffuse from stems/leaves to submerged roots.
- Supports aerobic respiration in roots despite low external oxygen.
Ethanol Fermentation in Roots
- When oxygen is very limited, roots perform anaerobic respiration.
- Glucose → ethanol + CO₂ + small ATP.
- Provides enough energy for root survival under waterlogging.
Faster Growth of Stems
- Stems elongate rapidly to keep leaves above water.
- Ensures photosynthesis continues efficiently.
- Facilitates oxygen transport through aerenchyma to submerged parts.
– Aerenchyma → oxygen transport.
– Ethanol fermentation → energy in low-oxygen roots.
– Rapid stem growth → maintains photosynthesis and oxygen supply.
Investigating Yeast Respiration Using Redox Indicators
📌 Overview
- Redox indicators like DCPIP and methylene blue are electron acceptors.
- They change color when reduced, allowing measurement of respiration rate.
- Useful for testing effects of temperature and substrate concentration on yeast metabolism.
🌱 Principle
- During aerobic respiration, electrons are transferred from substrates to coenzymes (NADH/FADH₂).
- Redox indicators act as artificial electron acceptors:
- DCPIP: Blue → Colorless when reduced.
- Methylene blue: Blue → Colorless when reduced.
- Rate of color change indicates rate of respiration.
🌱 Experimental Setup
- Prepare yeast suspension in a sugar solution (glucose, sucrose, etc.).
- Add redox indicator (DCPIP or methylene blue).
- Incubate under different conditions:
- Vary temperature (e.g., 20°C, 30°C, 40°C).
- Vary substrate concentration (e.g., 0.5%, 1%, 2% sugar).
- Observe or measure color change:
- Faster color loss → faster respiration.
- Optional: Measure absorbance with a colorimeter for quantitative results.
🔍 Variables
| Variable Type | Example |
|---|---|
| Independent | Temperature, substrate concentration |
| Dependent | Rate of color change (respiration rate) |
| Controlled | Yeast volume, indicator volume, pH, incubation time |
– Redox indicators provide a visual way to measure electron transfer during respiration.
– Optimal conditions (temperature, substrate) increase rate of respiration, seen as faster color change.
– Useful for comparing yeast activity under different environmental conditions.
Investigating the Effect of Temperature on Respiration Using Respirometers
📌 Overview
- Respirometers measure oxygen uptake or carbon dioxide production during respiration.
- Used to study how environmental factors like temperature affect metabolic rate.
- Commonly used with germinating seeds or small invertebrates (e.g., blowfly larvae).
🌱 Principle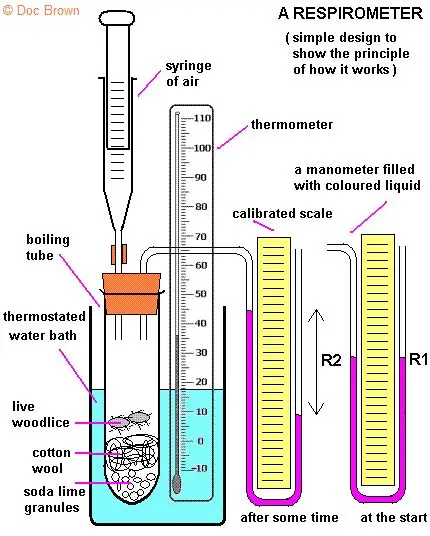
- Respiration consumes O₂ and produces CO₂ (in aerobic conditions).
- Oxygen uptake can be measured directly or via absorption of CO₂ (e.g., using potassium hydroxide).
- Rate of respiration is indicated by the volume of gas absorbed or displaced over time.
🌱 Experimental Setup
- Assemble simple respirometer:
- Test tube with respiring material (seeds/larvae) and control tube (non-respiring or boiled material).
- Place capillary tube with colored liquid to measure gas displacement.
- If CO₂ is absorbed (e.g., by KOH), movement of colored liquid reflects O₂ consumption.
- Vary temperature:
- Place respirometer in water baths at different temperatures (e.g., 10°C, 20°C, 30°C, 40°C).
- Record results:
- Measure displacement of liquid over time at each temperature.
- Faster movement → higher respiration rate.
🔍 Variables
| Variable Type | Example |
|---|---|
| Independent | Temperature of water bath |
| Dependent | Rate of respiration (liquid displacement) |
| Controlled | Volume of respirometer, mass of material, duration, CO₂ absorption method |
– Respiration rate increases with temperature up to an optimum, then decreases if enzymes denature.
– Simple respirometers provide a quantitative and visual method to study oxygen consumption.
– Using control respirometers ensures changes are due to biological activity and not physical factors.
