CIE AS/A Level Biology -2.1 Testing for biological molecules- Study Notes- New Syllabus
CIE AS/A Level Biology -2.1 Testing for biological molecules- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -2.1 Testing for biological molecules- Study Notes- New Syllabus
Key Concepts:
- describe and carry out the Benedict’s test for reducing sugars, the iodine test for starch, the emulsion test for lipids and the biuret test for proteins
- describe and carry out a semi-quantitative Benedict’s test on a reducing sugar solution by standardising the test and using the results (time to first colour change or comparison to colour standards) to estimate the concentration
- describe and carry out a test to identify the presence of non-reducing sugars, using acid hydrolysis and Benedict’s solution
Biochemical Tests for Macromolecules
🔬 Benedict’s Test for Reducing Sugars
- Purpose: Detects reducing sugars (e.g., glucose, fructose).
- Procedure:
- Add Benedict’s reagent (blue) to test solution.
- Heat gently in boiling water bath for 2–5 minutes.
- Positive Result: Color changes blue → green → yellow → orange → brick-red precipitate (more red = more sugar).
- Negative Result: Solution remains blue.
🟤 Iodine Test for Starch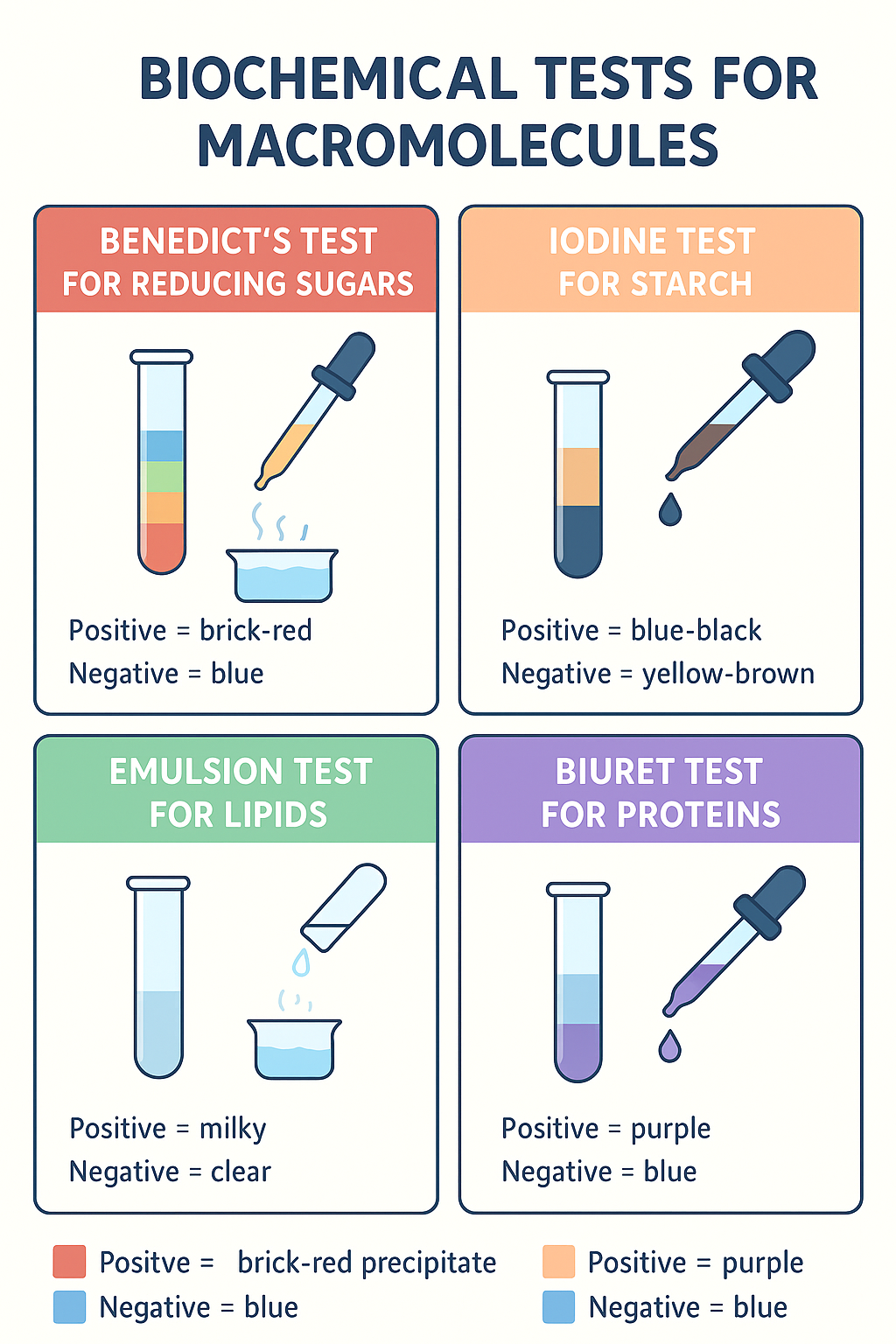
- Purpose: Detects presence of starch.
- Procedure:
- Add a few drops of iodine solution to sample.
- Positive Result: Color changes yellow-brown → blue-black or purple-black.
- Negative Result: No color change; stays yellow-brown.
💧 Emulsion Test for Lipids
- Purpose: Detects lipids (fats and oils).
- Procedure:
- Mix sample with ethanol and shake well.
- Add water and shake again.
- Positive Result: Cloudy white emulsion forms (milky layer).
- Negative Result: Solution remains clear.
🟣 Biuret Test for Proteins
- Purpose: Detects peptide bonds in proteins.
- Procedure:
- Add Biuret reagent (sodium hydroxide + copper sulfate) to sample.
- Mix gently.
- Positive Result: Color changes blue → violet or purple.
- Negative Result: Solution remains blue.
| Test | Detects | Key Reagent | Positive Result Color |
|---|---|---|---|
| Benedict’s Test | Reducing sugars | Benedict’s reagent | Blue → Brick-red precipitate |
| Iodine Test | Starch | Iodine solution | Yellow-brown → Blue-black |
| Emulsion Test | Lipids | Ethanol + Water | Clear → Cloudy white emulsion |
| Biuret Test | Proteins | Biuret reagent | Blue → Violet/Purple |
🧠 Summary Box:
Biochemical tests use specific reagents that cause distinct color changes to identify reducing sugars, starch, lipids, and proteins.
Biochemical tests use specific reagents that cause distinct color changes to identify reducing sugars, starch, lipids, and proteins.
Semi-Quantitative Benedict’s Test for Reducing Sugars
🌱 Purpose
- To estimate the concentration of reducing sugars in a solution by measuring how quickly a color change occurs or by comparing the color intensity to known standards.
🔍 Standardising the Benedict’s Test
- Prepare Standard Solutions: Make a series of reducing sugar solutions of known concentrations (e.g., 0.1%, 0.2%, 0.5%, 1%, 2%).
- Add Benedict’s Reagent: To each standard solution, add an equal volume of Benedict’s reagent.
- Heat: Heat the test tubes in a boiling water bath for a fixed time (e.g., 5 minutes).
- Observe Colour Change: Record the color of each solution after heating (blue, green, yellow, orange, brick-red).
- Create a Colour Standard Chart: Arrange the colors corresponding to increasing sugar concentrations as a reference.
🧪 Carrying Out the Semi-Quantitative Test
- Test Unknown Sample: Add Benedict’s reagent to the unknown reducing sugar solution in a test tube.
- Heat and Time: Heat in a boiling water bath and start a stopwatch.
- Record Time of First Colour Change: Note the time taken for the solution to change from blue to the first sign of any color (green/yellow/orange/red).
- Compare with Standards: Estimate concentration by:
- Comparing the time taken for colour change with times from standard solutions.
- Or matching the final colour intensity to the colour standard chart.
📊 Interpreting Results
| Time to First Colour Change | Approximate Reducing Sugar Concentration | Colour After 5 Minutes |
|---|---|---|
| >5 minutes (no change) | Very low or zero | Blue (no precipitate) |
| ~4-5 minutes | Low concentration | Green-yellow |
| ~2-4 minutes | Moderate concentration | Orange |
| <2 minutes | High concentration | Brick-red precipitate |
⚠️ Important Notes
- Always use the same volumes and heating time for consistency.
- The test is semi-quantitative-it gives an estimate, not an exact measurement.
- Use color charts and timing carefully to reduce subjective errors.
🧠 Summary
The quicker the color change or the more intense the colour after heating, the higher the concentration of reducing sugars.
Standardisation with known concentrations allows comparison and estimation.
The quicker the color change or the more intense the colour after heating, the higher the concentration of reducing sugars.
Standardisation with known concentrations allows comparison and estimation.
Test for Non-Reducing Sugars Using Acid Hydrolysis and Benedict’s Solution
🌱 Purpose
- To detect non-reducing sugars (e.g., sucrose) which do not react directly with Benedict’s reagent unless first broken down into reducing sugars.
🔍 Principle
- Non-reducing sugars do not reduce Benedict’s reagent initially.
- When heated with dilute acid (acid hydrolysis), non-reducing sugars break down into reducing sugars.
- After neutralising the acid, Benedict’s test is repeated to detect the presence of reducing sugars formed.
🧪 Materials Needed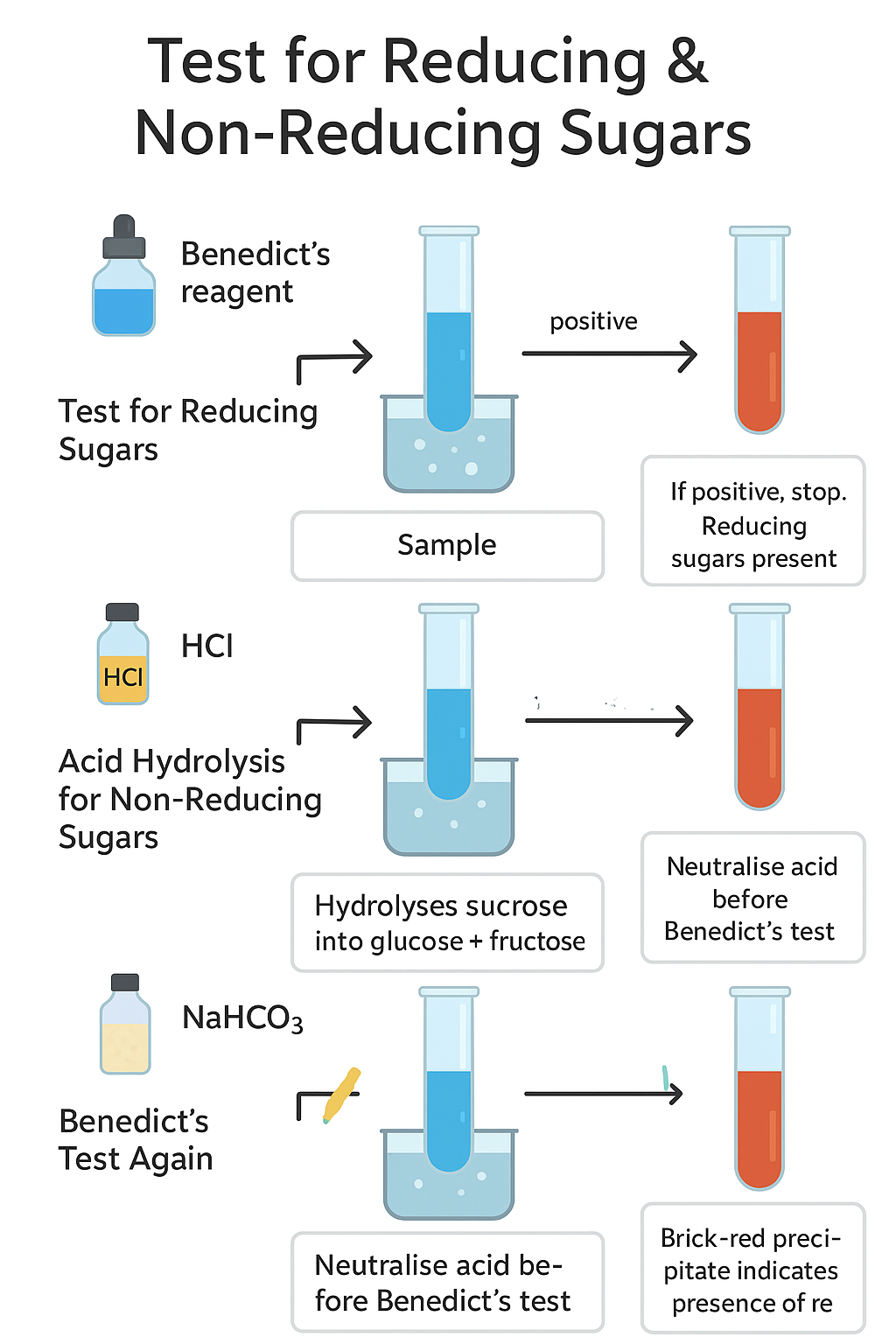
- Sample solution
- Benedict’s reagent
- Dilute hydrochloric acid (HCl)
- Sodium hydrogen carbonate (NaHCO₃) or sodium hydroxide (NaOH) for neutralisation
- Boiling water bath
- Test tubes
🔬 Procedure
- Test for Reducing Sugars (Control):
- Add Benedict’s reagent to a sample of the solution.
- Heat in boiling water bath for 2–5 minutes.
- If positive (color change), reducing sugars are present; test for non-reducing sugars is not needed.
- If negative (no color change), proceed to Step 2.
- Acid Hydrolysis of Non-Reducing Sugars:
- Take a fresh sample of the test solution in a test tube.
- Add a few drops of dilute hydrochloric acid.
- Heat gently in a boiling water bath for 2 minutes to hydrolyse the non-reducing sugars into reducing sugars.
- Neutralisation:
- Carefully neutralise the acid by adding sodium hydrogen carbonate (or NaOH) dropwise until fizzing stops and the solution is neutral (test with pH paper if available).
- Benedict’s Test on Hydrolysed Sample:
- Add Benedict’s reagent to the neutralised solution.
- Heat again in the boiling water bath for 2–5 minutes.
🔍 Results Interpretation
| Observation | Conclusion |
|---|---|
| No color change in Step 1, but colour changes to brick-red in Step 4 | Non-reducing sugars are present after acid hydrolysis and Benedict’s test |
| No colour change in both steps | No reducing or non-reducing sugars present |
🧠 Summary
Non-reducing sugars do not react with Benedict’s reagent initially.
Acid hydrolysis breaks non-reducing sugars into reducing sugars.
After neutralisation, Benedict’s test detects the newly formed reducing sugars.
This confirms the presence of non-reducing sugars in the original sample.
Non-reducing sugars do not react with Benedict’s reagent initially.
Acid hydrolysis breaks non-reducing sugars into reducing sugars.
After neutralisation, Benedict’s test detects the newly formed reducing sugars.
This confirms the presence of non-reducing sugars in the original sample.
