CIE AS/A Level Biology -2.2 Carbohydrates and lipids- Study Notes- New Syllabus
CIE AS/A Level Biology -2.2 Carbohydrates and lipids- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -2.2 Carbohydrates and lipids- Study Notes- New Syllabus
Key Concepts:
- describe and draw the ring forms of α-glucose and β-glucose
- define the terms monomer, polymer, macromolecule, monosaccharide, disaccharide and polysaccharide
- state the role of covalent bonds in joining smaller molecules together to form polymers
- state that glucose, fructose and maltose are reducing sugars and that sucrose is a non-reducing sugar
- describe the formation of a glycosidic bond by condensation, with reference to disaccharides, including sucrose, and polysaccharides
- describe the breakage of a glycosidic bond in polysaccharides and disaccharides by hydrolysis, with reference to the non-reducing sugar test
- describe the molecular structure of the polysaccharides starch (amylose and amylopectin) and glycogen and relate their structures to their functions in living organisms
- describe the molecular structure of the polysaccharide cellulose and outline how the arrangement of cellulose molecules contributes to the function of plant cell walls
- state that triglycerides are non-polar hydrophobic molecules and describe the molecular structure of triglycerides with reference to fatty acids (saturated and unsaturated), glycerol and the formation of ester bonds
- relate the molecular structure of triglycerides to their functions in living organisms
- describe the molecular structure of phospholipids with reference to their hydrophilic (polar) phosphate heads and hydrophobic (non-polar) fatty acid tails
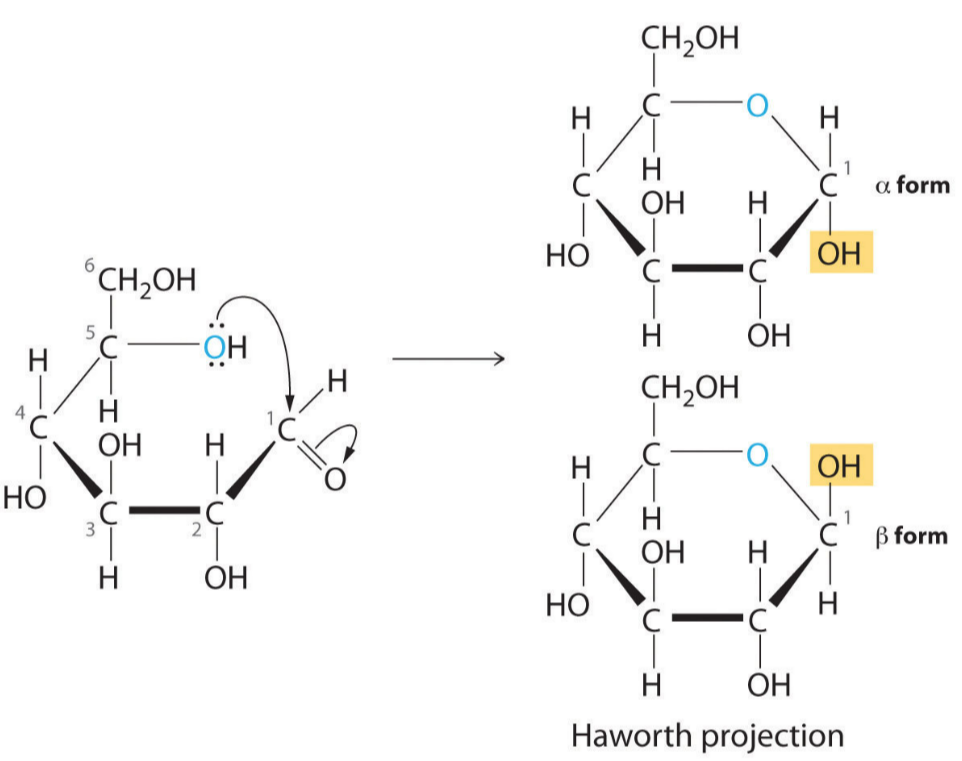
Ring Forms of α-Glucose and β-Glucose
🌱 Description of α-Glucose and β-Glucose
- Both α-glucose and β-glucose are isomers of glucose with the molecular formula C₆H₁₂O₆.
- They differ in the position of the -OH group attached to carbon 1 (the anomeric carbon) in the ring form.
- These forms exist because glucose cyclizes (forms a ring) in aqueous solutions, creating a hemiacetal ring structure (a six-membered pyranose ring).
🔍 Key Structural Difference
| Feature | α-Glucose | β-Glucose |
|---|---|---|
| -OH group on carbon 1 (anomeric carbon) | Points down (below the plane of the ring) | Points up (above the plane of the ring) |
| Common form in nature | Found in starch and glycogen | Found in cellulose |
🧪 Structure Details
- The ring is formed by bonding carbon 1 and carbon 5 oxygen to form a hexagonal pyranose ring.
- Carbon atoms are numbered clockwise from the oxygen in the ring.
- The difference between α and β is crucial because it affects how glucose polymers (like starch and cellulose) form.
✍️Diagram of α-Glucose and β-Glucose (Haworth Projections)
Note:
In α-glucose, the -OH on carbon 1 points down (same side as C6 CH₂OH group).
In β-glucose, the -OH on carbon 1 points up (opposite side to C6 CH₂OH group).
📌 Summary
| Isomer | OH on C1 Position | Role in Polysaccharides |
|---|---|---|
| α-Glucose | Down | Building block of starch, glycogen |
| β-Glucose | Up | Building block of cellulose |
Definitions of Key Biological Terms
🌱 Monomer
- A monomer is a small, simple molecule that can join with other similar molecules to form a larger molecule called a polymer.
- Example: Glucose is a monomer for carbohydrates.
🌿 Polymer
- A polymer is a large molecule made up of many repeating monomers linked together.
- Example: Starch is a polymer made from glucose monomers.
🧪 Macromolecule
- A macromolecule is a very large molecule essential to life, often made by polymerising smaller units (monomers).
- Includes carbohydrates, proteins, lipids, and nucleic acids.
🍬 Monosaccharide
- The simplest form of carbohydrate; a single sugar unit (monomer).
- Examples: Glucose, fructose, galactose.
🍭 Disaccharide
- A carbohydrate made of two monosaccharide units joined by a glycosidic bond.
- Examples: Sucrose (glucose + fructose), lactose (glucose + galactose).
🍞 Polysaccharide
- A carbohydrate polymer made of many monosaccharide units linked together.
- Examples: Starch, glycogen, cellulose.
📊 Summary Table
| Term | Definition | Example |
|---|---|---|
| Monomer | Small molecule that can join to form polymers | Glucose |
| Polymer | Large molecule made of repeating monomers | Starch |
| Macromolecule | Very large molecule essential to life | Proteins, carbohydrates |
| Monosaccharide | Single sugar unit | Glucose |
| Disaccharide | Two monosaccharides joined | Sucrose |
| Polysaccharide | Many monosaccharides joined | Cellulose, glycogen |
Role of Covalent Bonds in Forming Polymers
🌱 Key Role of Covalent Bonds
- Covalent bonds are strong chemical bonds formed by the sharing of electron pairs between atoms.
- They join smaller molecules (monomers) together to form larger molecules called polymers.
- During polymerisation, covalent bonds form between monomers by condensation reactions, where a small molecule (often water) is removed.
- These covalent bonds create stable, strong linkages that hold the polymer chain together.
🔍 Example
- In carbohydrates, covalent glycosidic bonds join monosaccharides.
- In proteins, peptide bonds (a type of covalent bond) link amino acids.
- In nucleic acids, phosphodiester bonds join nucleotides.
Covalent bonds are essential for building stable, long chains of polymers from smaller monomers.
These bonds determine the structure and properties of biological macromolecules.
Reducing and Non-Reducing Sugars
🌱 Reducing Sugars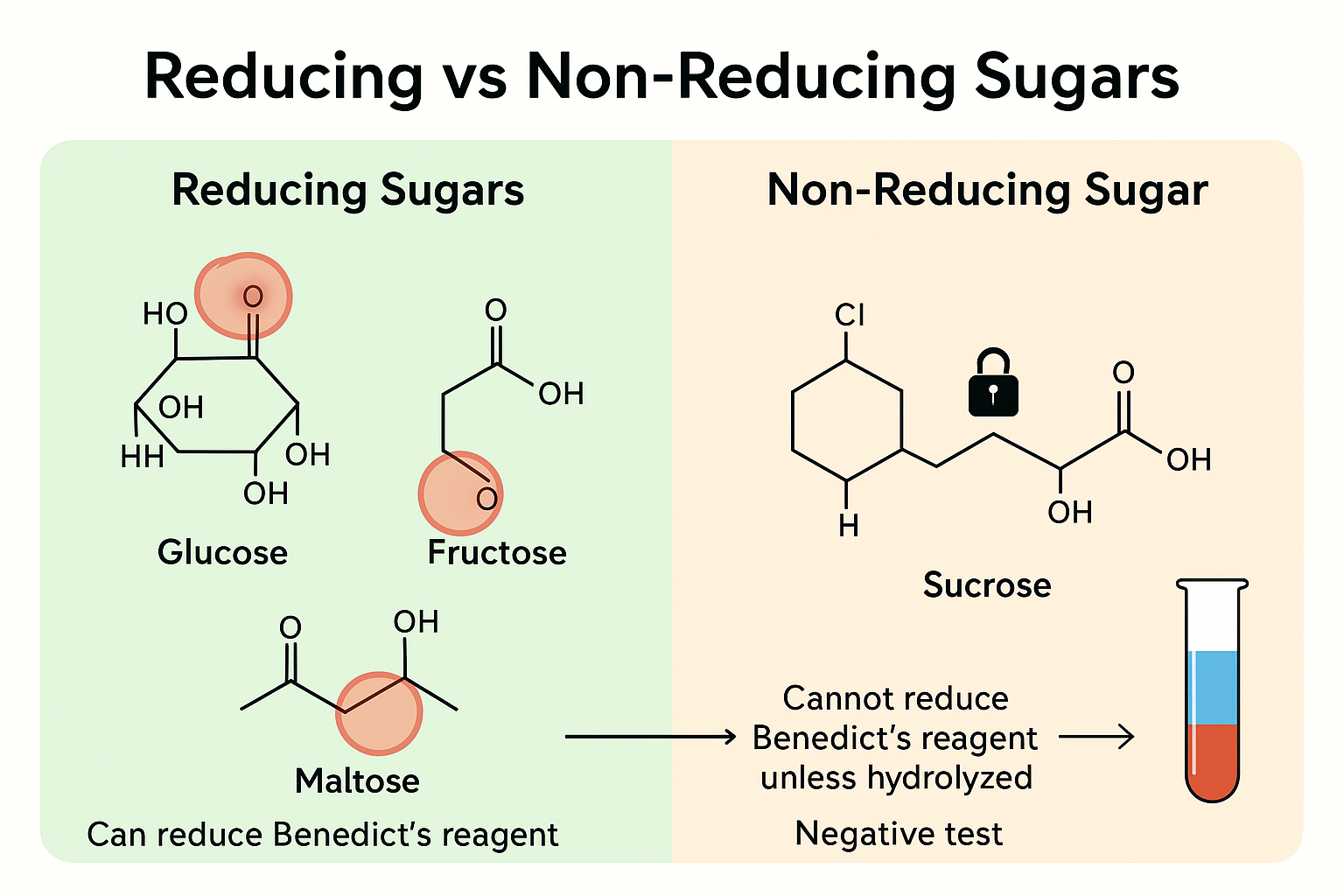
- Glucose, fructose, and maltose are reducing sugars.
- They have free aldehyde or ketone groups that can donate electrons and reduce other substances like Benedict’s reagent.
- This property allows them to participate in redox reactions.
🌿 Non-Reducing Sugar
- Sucrose is a non-reducing sugar.
- Its glycosidic bond blocks the reactive group, so it cannot reduce Benedict’s reagent directly.
- Sucrose does not give a positive Benedict’s test unless it is first broken down by hydrolysis.
| Sugar | Reducing or Non-Reducing | Reason |
|---|---|---|
| Glucose | Reducing | Has free aldehyde group |
| Fructose | Reducing | Has free ketone group |
| Maltose | Reducing | Has free aldehyde group after bond cleavage |
| Sucrose | Non-reducing | Glycosidic bond blocks reactive group |
Formation of a Glycosidic Bond by Condensation
🌱 What is a Glycosidic Bond?
- A glycosidic bond is a covalent bond that links two sugar molecules (monosaccharides).
- It forms when two hydroxyl groups (-OH) on adjacent sugar molecules react.
🔬 Condensation Reaction
- During glycosidic bond formation, a condensation reaction occurs:
- A molecule of water (H₂O) is removed (lost).
- The oxygen atom forms a bridge between the two sugar units, creating the glycosidic bond.
🍭 In Disaccharides
- Example: Sucrose is formed from glucose and fructose by a glycosidic bond.
- The bond forms between carbon 1 of glucose and carbon 2 of fructose, releasing water.
🌿 In Polysaccharides
- Polysaccharides (e.g., starch, glycogen, cellulose) are long chains of monosaccharides linked by many glycosidic bonds formed through repeated condensation reactions.
- The type of glycosidic bond (e.g., α-1,4 or β-1,4) affects the structure and properties of the polysaccharide.
| Process | Description |
|---|---|
| Glycosidic Bond Formation | Covalent bond formed between two sugars |
| Reaction Type | Condensation (water molecule removed) |
| Example (Disaccharide) | Sucrose: glucose + fructose linked by bond |
| Example (Polysaccharide) | Starch: many glucose units linked by glycosidic bonds |
Glycosidic bonds join sugar units into larger carbohydrates by condensation, crucial for energy storage and structural molecules in cells.
Breakage of Glycosidic Bonds by Hydrolysis
🌱 What is Hydrolysis?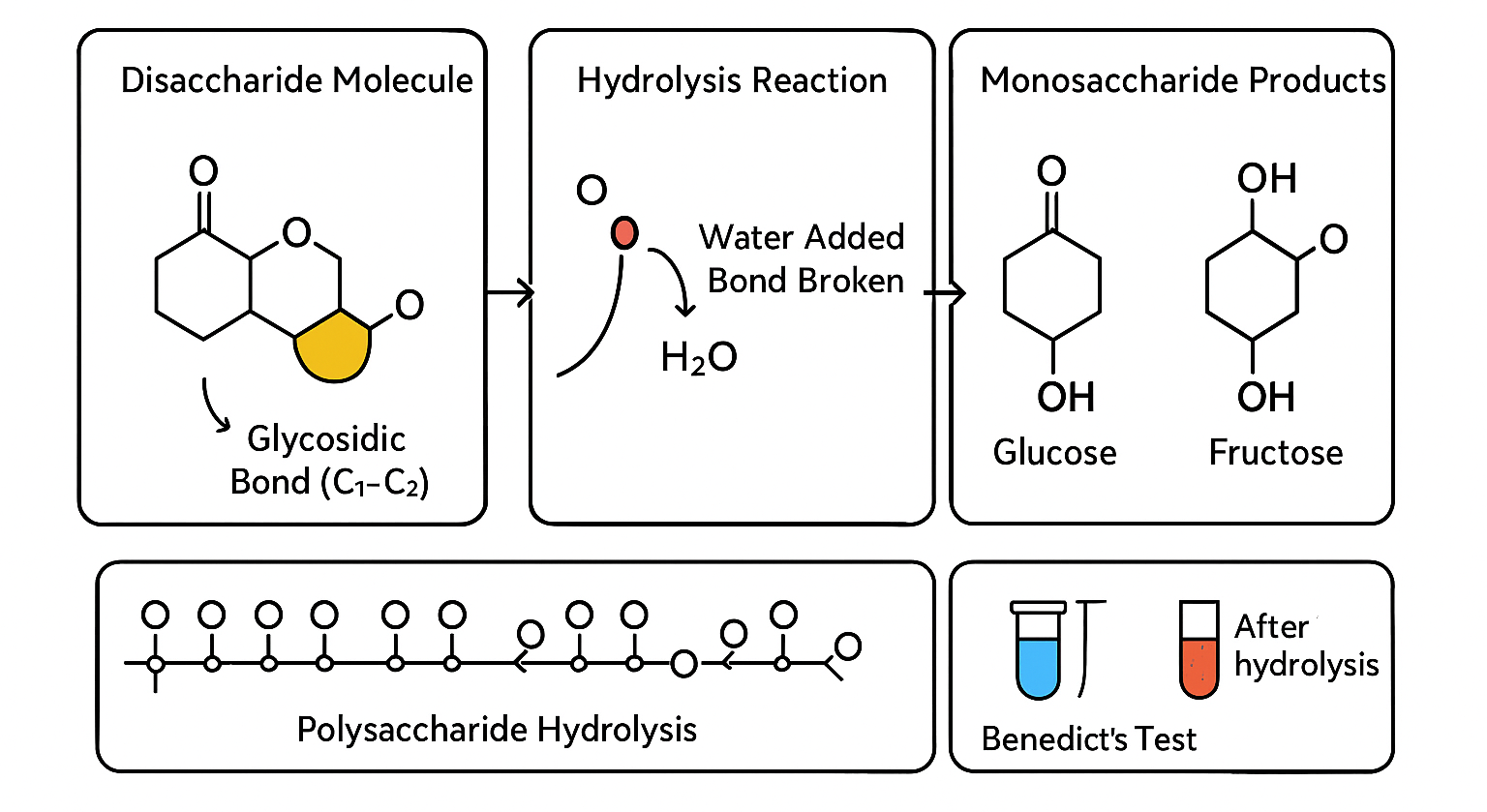
- Hydrolysis is the process of breaking chemical bonds by adding a water molecule (H₂O).
- It is the reverse of condensation.
🔍 Hydrolysis of Glycosidic Bonds
- In carbohydrates, hydrolysis breaks glycosidic bonds between sugar units in disaccharides and polysaccharides.
- This splits larger sugars into smaller sugars or monosaccharides.
- The addition of water breaks the bond, releasing the individual sugar units.
🍭 Example in Disaccharides and Polysaccharides
- Disaccharides like sucrose can be hydrolysed into glucose and fructose.
- Polysaccharides like starch and cellulose can be broken down into many glucose molecules by hydrolysis.
🧪 Relation to Non-Reducing Sugar Test
- Some sugars (e.g., sucrose) are non-reducing sugars and do not react with Benedict’s reagent initially because their glycosidic bonds block the reactive group.
- When hydrolysed (using dilute acid or enzymes), these sugars break into reducing sugars (e.g., glucose and fructose).
- After hydrolysis and neutralisation, the Benedict’s test becomes positive, indicating reducing sugars are present.
| Process | Description | Example |
|---|---|---|
| Hydrolysis | Breaking glycosidic bonds by adding water | Sucrose → Glucose + Fructose |
| Effect on Tests | Converts non-reducing sugars to reducing sugars | Positive Benedict’s after hydrolysis |
Molecular Structure and Function of Polysaccharides: Starch and Glycogen
🌱 Starch (Energy Storage in Plants)
Starch is a storage polysaccharide in plants made of two components:
- Amylose
Structure:- Long, unbranched chains of α-glucose units.
- Glucose molecules linked by α-1,4 glycosidic bonds.
- Forms a helical (coiled) structure.
Function:
- Compact helical shape makes amylose efficient for energy storage.
- Insoluble in water, so it doesn’t affect cell water balance.
- Amylopectin
Structure:- Branched chains of α-glucose.
- Has α-1,4 glycosidic bonds along the chain and α-1,6 glycosidic bonds at branch points every ~25 glucose units.
Function:
- Branching provides many ends for enzyme action, speeding up glucose release during respiration.
🌿 Glycogen (Energy Storage in Animals and Fungi)
- Structure:
- Similar to amylopectin but more highly branched (branches every 8–12 glucose units).
- Made of α-glucose linked by α-1,4 bonds (chains) and α-1,6 bonds (branches).
- Function:
- High branching allows rapid release of glucose to meet sudden energy demands.
- Compact and insoluble, making it ideal for storage without affecting cell water potential.
| Feature | Starch (Amylose & Amylopectin) | Glycogen |
|---|---|---|
| Monomer | α-Glucose | α-Glucose |
| Chain Type | Amylose: unbranched; Amylopectin: moderately branched | Highly branched |
| Bond Types | α-1,4 (chains), α-1,6 (branches in amylopectin) | α-1,4 (chains), α-1,6 (branches) |
| Branching Frequency | Less frequent (~25 units) | More frequent (~8–12 units) |
| Function | Energy storage in plants | Energy storage in animals and fungi |
| Structural Role | Compact, helical structure in amylose; easier enzyme access in amylopectin | Rapid glucose release due to many branch ends |
Starch is the main plant energy store, combining compact amylose and branched amylopectin for storage and quick energy release.
Glycogen is the animal energy store, highly branched for faster glucose availability.
Their molecular structures directly influence how effectively organisms store and mobilize energy.
Molecular Structure and Function of Cellulose in Plant Cell Walls
🌱 Molecular Structure of Cellulose
- Cellulose is a polysaccharide made of many β-glucose units.
- Glucose monomers are linked by β-1,4 glycosidic bonds.
- Each adjacent glucose unit is rotated 180°, creating a straight, unbranched chain.
- Many cellulose chains align parallel to each other and form strong microfibrils through hydrogen bonds between hydroxyl (-OH) groups on adjacent chains.
🔍 Arrangement and Function
- The microfibrils bundle tightly to form a rigid network.
- This structure provides high tensile strength, preventing the plant cell from bursting under turgor pressure.
- The cellulose network is insoluble and stable, giving structural support and rigidity to plant cell walls.
- Allows plants to maintain shape and stand upright.
| Feature | Description |
|---|---|
| Monomer | β-glucose |
| Bond Type | β-1,4 glycosidic bonds |
| Chain Structure | Straight, unbranched chains |
| Interactions | Hydrogen bonds between chains |
| Higher Structure | Microfibrils and fibres |
| Function | Provides strength and rigidity to plant cell walls |
The straight chains and hydrogen bonding between cellulose molecules form strong microfibrils, crucial for the mechanical strength and protection of plant cells.
Triglycerides: Structure and Properties
🌱 Key Properties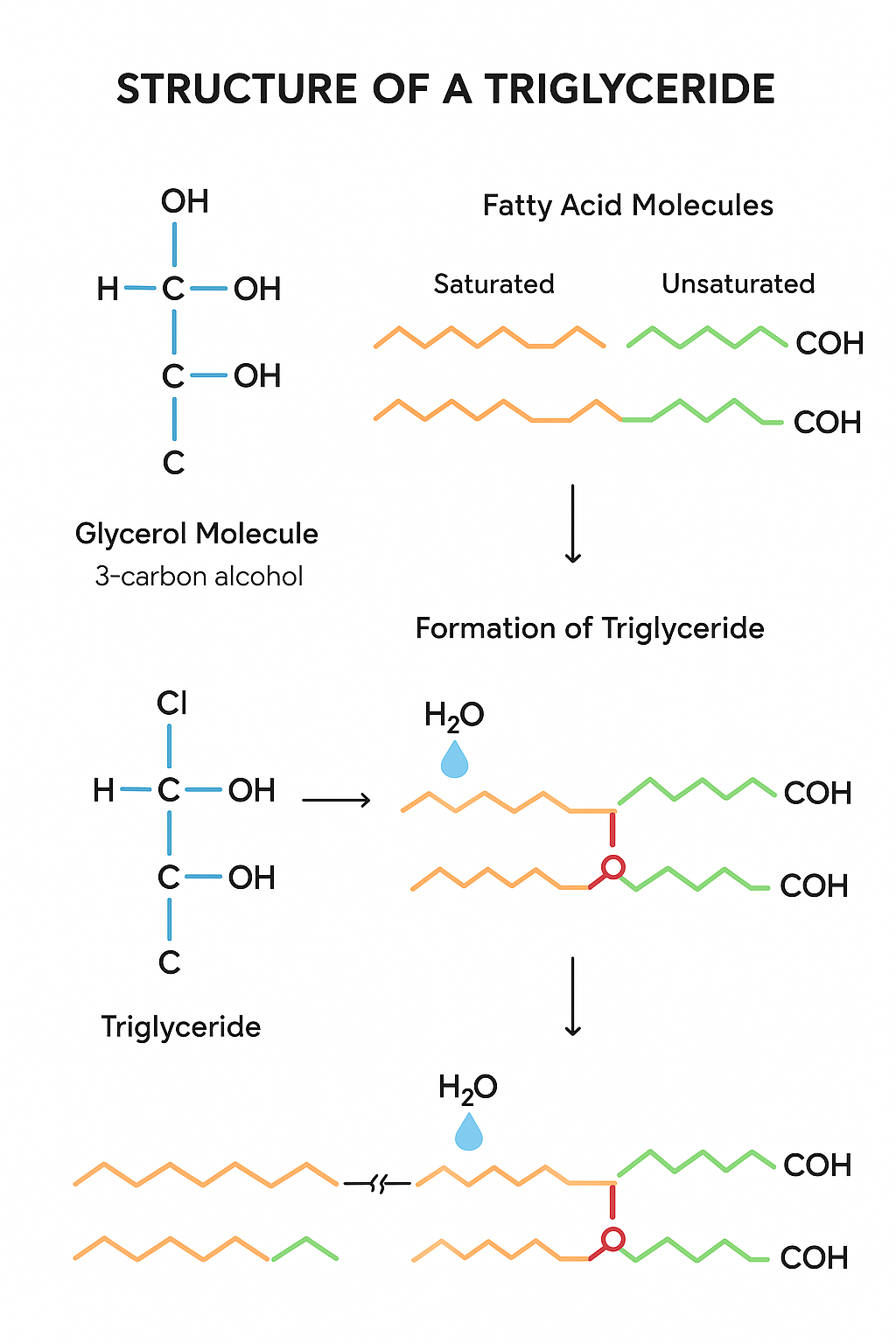
- Triglycerides are non-polar, hydrophobic molecules.
- They do not mix with water because they lack charged (polar) groups.
🔍 Molecular Structure of Triglycerides
- A triglyceride molecule consists of:
- One glycerol molecule (a 3-carbon alcohol with three –OH groups).
- Three fatty acid molecules, each attached to glycerol.
🍃 Fatty Acids
- Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end.
- Types of fatty acids:
- Saturated fatty acids: No double bonds between carbon atoms; chains are straight.
- Unsaturated fatty acids: One or more double bonds; chains have kinks (bends).
🔗 Formation of Ester Bonds
- Each fatty acid joins to glycerol by a condensation reaction.
- An ester bond forms between the carboxyl group of the fatty acid and a hydroxyl group (-OH) of glycerol, releasing water.
- This results in three ester bonds per triglyceride molecule.
| Component | Description |
|---|---|
| Glycerol | 3-carbon molecule with –OH groups |
| Fatty acids | Long hydrocarbon chains (saturated or unsaturated) |
| Bond type | Ester bonds (formed by condensation) |
| Property | Non-polar and hydrophobic |
The non-polar hydrocarbon tails of fatty acids make triglycerides hydrophobic, ideal for energy storage without affecting water balance in cells.
Relationship Between Molecular Structure of Triglycerides and Their Functions
🌱 Key Structural Features of Triglycerides
- Composed of one glycerol molecule and three fatty acids linked by ester bonds.
- Fatty acids have long non-polar hydrocarbon tails, making triglycerides hydrophobic (water-insoluble).
- Fatty acids can be saturated (straight chains) or unsaturated (kinked chains).
🔍 How Structure Relates to Function
| Structural Feature | Functional Benefit |
|---|---|
| Hydrophobic nature | Triglycerides form energy-dense fat stores that do not dissolve in water, preventing osmotic problems inside cells. |
| Long hydrocarbon chains | Provide a high amount of chemical energy when broken down (more than carbohydrates). |
| Ester bonds | Allow stable storage of fatty acids and release via hydrolysis when energy is needed. |
| Saturated vs unsaturated tails | Saturated fats pack tightly for solid energy storage; unsaturated fats provide fluidity and prevent packing, influencing membrane properties and energy use. |
| Compact structure | Efficient storage of energy in small volume, important for animals with limited space. |
| Insulation and protection | Stored fat in organisms provides thermal insulation and cushioning for organs. |
The non-polar, hydrophobic nature of triglycerides makes them excellent for long-term energy storage without interfering with water balance.
Their energy-rich hydrocarbon chains supply more than twice the energy per gram compared to carbohydrates.
Their compact, stable structure supports energy storage, insulation, and protection in living organisms.
Molecular Structure of Phospholipids
🌱 Key Components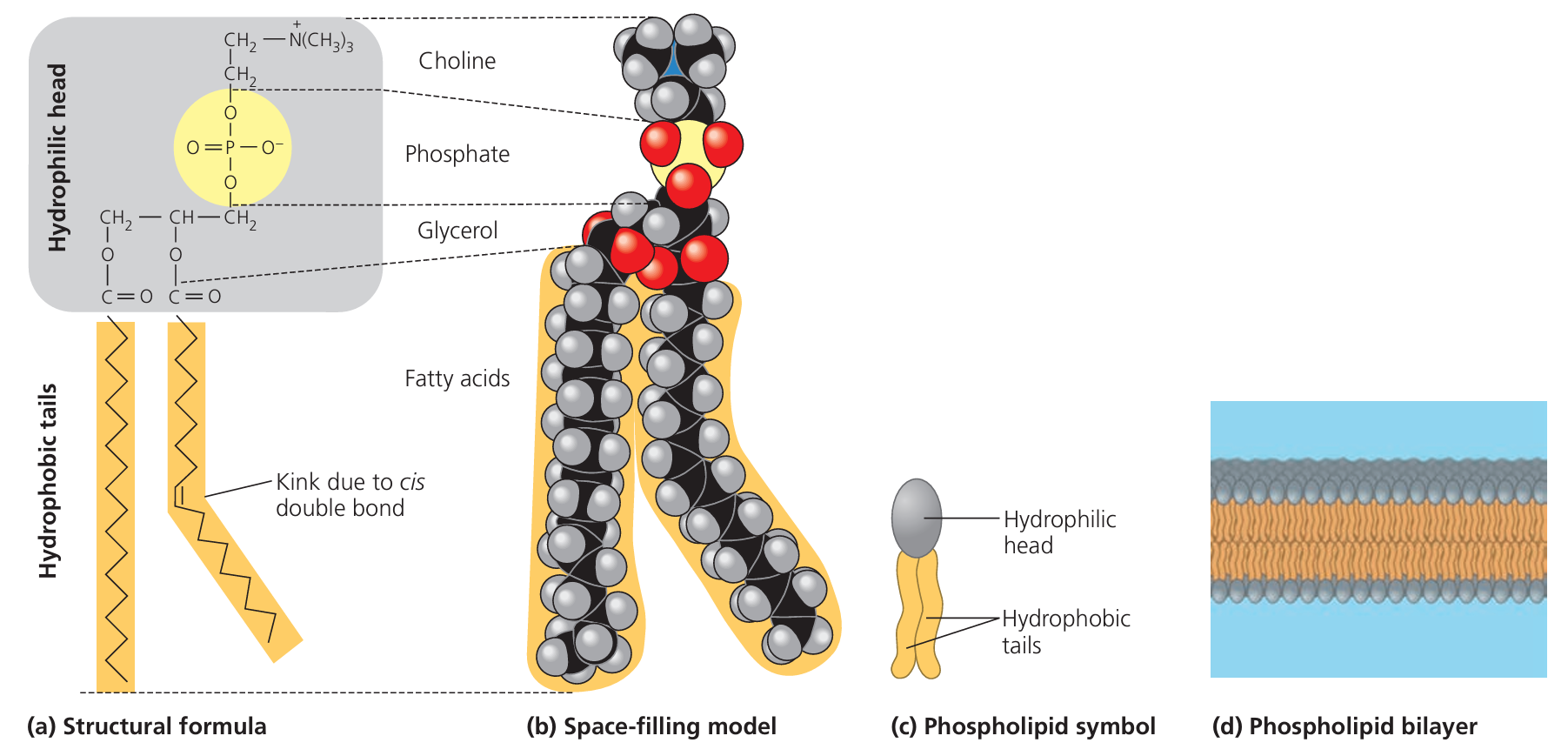
- One glycerol molecule (3-carbon alcohol).
- Two fatty acid tails (long hydrocarbon chains).
- One phosphate group attached to the glycerol.
🔍 Hydrophilic Head
- The phosphate group is polar and hydrophilic (“water-loving”).
- This head interacts readily with water because it carries a negative charge, making it soluble in water.
🔍 Hydrophobic Tails
- The two fatty acid tails are non-polar and hydrophobic (“water-fearing”).
- These long hydrocarbon chains repel water and tend to avoid contact with aqueous environments.
🔄 Overall Structure
- Phospholipids are amphipathic molecules (having both hydrophilic and hydrophobic parts).
- This dual nature causes them to arrange into bilayers in water, with heads facing outward toward water and tails inward, away from water.
| Part | Nature | Property | Function in Structure |
|---|---|---|---|
| Phosphate Head | Polar | Hydrophilic | Interacts with aqueous environments |
| Fatty Acid Tails | Non-polar | Hydrophobic | Avoids water; forms bilayer core |
The hydrophilic heads and hydrophobic tails enable phospholipids to form the cell membrane’s bilayer, providing a selective barrier in cells.
