CIE AS/A Level Biology -2.3 Proteins- Study Notes- New Syllabus
CIE AS/A Level Biology -2.3 Proteins- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -2.3 Proteins- Study Notes- New Syllabus
Key Concepts:
- describe and draw the general structure of an amino acid and the formation and breakage of a peptide bond
- explain the meaning of the terms primary structure, secondary structure, tertiary structure and quaternary structure of proteins
- describe the types of interaction that hold protein molecules in shape:
• hydrophobic interactions
• hydrogen bonding
• ionic bonding
• covalent bonding, including disulfide bonds - state that globular proteins are generally soluble and have physiological roles and fibrous proteins are generally insoluble and have structural roles
- describe the structure of a molecule of haemoglobin as an example of a globular protein, including the formation of its quaternary structure from two alpha (α) chains (α–globin), two beta (β) chains (β–globin) and a haem group
- relate the structure of haemoglobin to its function, including the importance of iron in the haem group
- describe the structure of a molecule of collagen as an example of a fibrous protein, and the arrangement of collagen molecules to form collagen fibres
- relate the structures of collagen molecules and collagen fibres to their function
General Structure of an Amino Acid and Peptide Bond Formation
🌱 General Structure of an Amino Acid
- Central carbon atom (alpha carbon, C).
- Amino group (-NH₂) attached to alpha carbon.
- Carboxyl group (-COOH) attached to alpha carbon.
- Hydrogen atom (H) attached to alpha carbon.
- R group (side chain) varies between amino acids, determining properties.
✍️ Diagram of a General Amino Acid
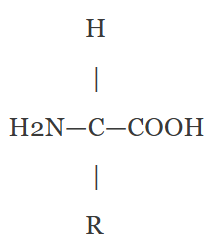
The R group varies (e.g., -CH₃ in alanine, -OH in serine).
🔗 Formation of a Peptide Bond (Condensation Reaction)
- Peptide bond is a covalent bond linking two amino acids.
- Forms between the carboxyl group (-COOH) of one amino acid and amino group (-NH₂) of another.
- Condensation reaction removes a water molecule (H₂O).
- Hydroxyl (–OH) from carboxyl and hydrogen (H) from amino combine to form water.
- The bond links carbon (C) of carboxyl to nitrogen (N) of amino group.
🔍 Peptide Bond Breakage (Hydrolysis Reaction)
- Peptide bonds can be broken by hydrolysis (addition of water).
- This splits the bond and releases individual amino acids.
- This is the reverse of condensation.
✍️ Diagram of Peptide Bond Formation
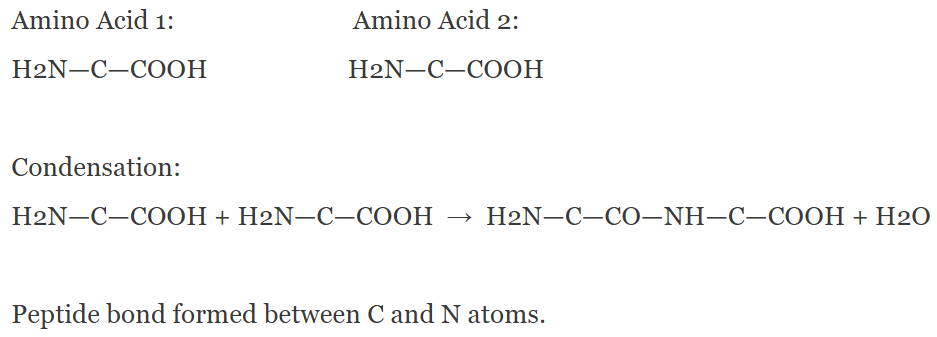
| Process | Description |
|---|---|
| Peptide Bond | Covalent bond linking amino acids via condensation (water removed) |
| Hydrolysis | Breaking peptide bonds by adding water |
| Amino Acid Parts | Central C, amino group, carboxyl group, H, R side chain |
Levels of Protein Structure
🌱 Primary Structure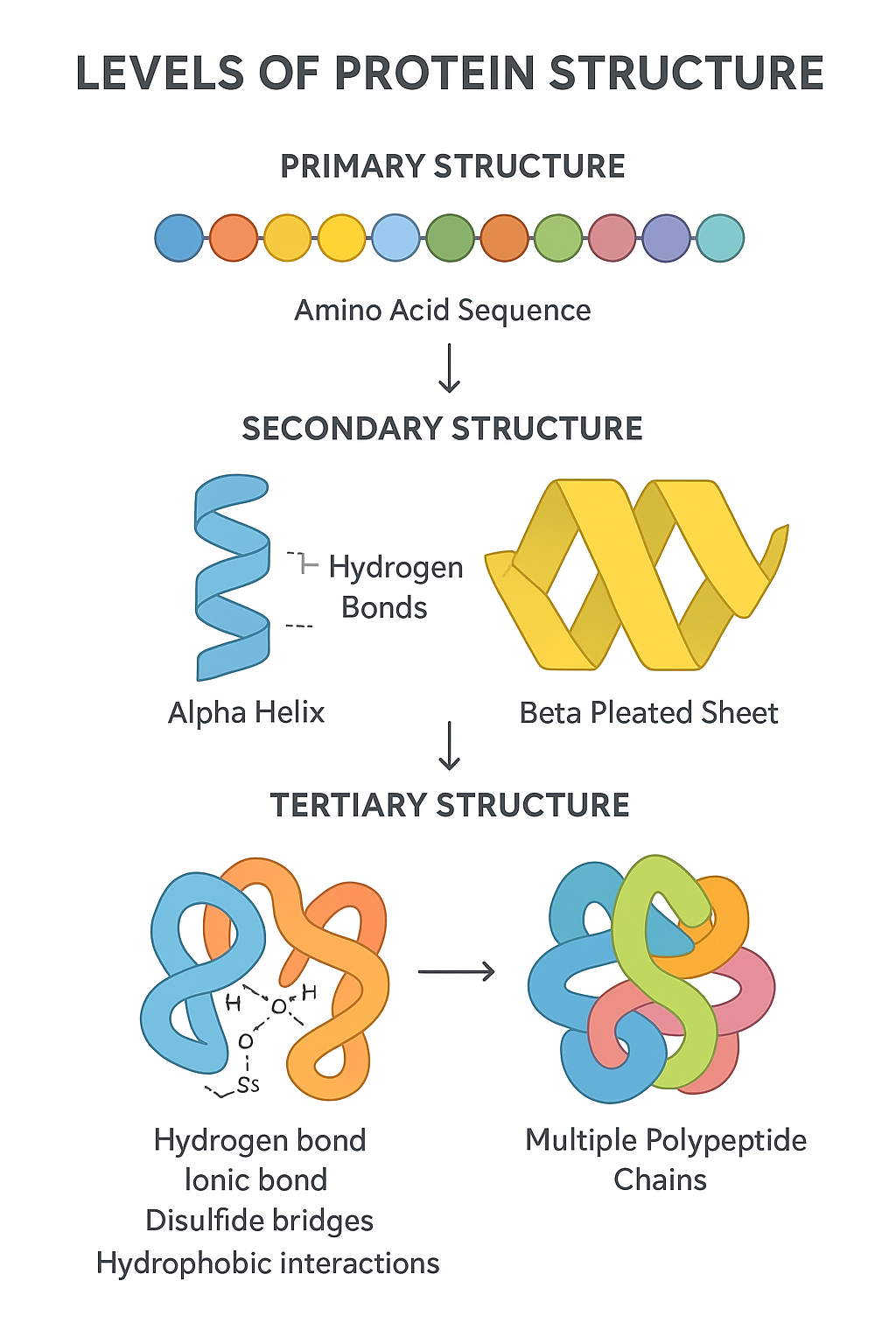
- Unique linear sequence of amino acids in a polypeptide chain.
- Determined by the gene encoding the protein.
- The amino acid order dictates all higher structure levels and protein function.
🌿 Secondary Structure
- Local folding of the polypeptide chain into regular shapes.
- Formed mainly by hydrogen bonds between backbone atoms (not side chains).
- Common forms:
- Alpha (α) helix — coiled spiral shape.
- Beta (β) pleated sheet — folded sheet-like structure.
🔬 Tertiary Structure
- Overall 3D shape of a single polypeptide chain.
- Formed by interactions between amino acid R groups (side chains), including:
- Hydrogen bonds
- Ionic bonds
- Disulfide bridges (covalent bonds between cysteines)
- Hydrophobic interactions
- Determines protein specificity and function.
🧠 Quaternary Structure
- Arrangement of multiple polypeptide chains (subunits) into a functional protein complex.
- Stabilized by the same bonds as tertiary structure.
- Examples: Hemoglobin (4 subunits), insulin (2 subunits).
| Level of Structure | Description | Key Features |
|---|---|---|
| Primary | Sequence of amino acids | Peptide bonds |
| Secondary | Local folding into α-helix or β-sheet | Hydrogen bonds (backbone) |
| Tertiary | 3D shape of one polypeptide | Interactions between side chains |
| Quaternary | Multiple polypeptides forming complex | Subunit interactions |
Types of Interactions That Hold Protein Molecules in Shape
🌱 1. Hydrophobic Interactions
- Occur between non-polar (hydrophobic) side chains of amino acids.
- Hydrophobic groups cluster inside the protein, away from water, stabilizing the 3D shape.
- Helps proteins fold by pushing hydrophobic regions inward.
🌿 2. Hydrogen Bonding
- Form between polar side chains or backbone atoms.
- A hydrogen atom is attracted to electronegative atoms like oxygen or nitrogen.
- Maintains secondary structures (α-helices and β-sheets) and stabilizes tertiary structure.
🔬 3. Ionic Bonding (Salt Bridges)
- Occurs between positively charged (basic) and negatively charged (acidic) side chains.
- Electrostatic attractions stabilize tertiary and quaternary structures.
- Sensitive to pH changes which can disrupt these bonds.
🔗 4. Covalent Bonding (Including Disulfide Bonds)
- The strongest bonds stabilizing protein structure.
- Disulfide bonds form between sulfur atoms of two cysteine residues.
- Provide strong links maintaining tertiary or quaternary structure, especially in extracellular proteins.
| Interaction | Description | Role in Protein Structure |
|---|---|---|
| Hydrophobic | Non-polar side chains cluster inside protein | Drives folding by avoiding water |
| Hydrogen bonds | Between polar groups (backbone or side chains) | Stabilizes secondary and tertiary structure |
| Ionic bonds | Between charged side chains | Stabilizes tertiary/quaternary, pH sensitive |
| Covalent bonds | Strong bonds including disulfide bonds (S–S) | Provides strong, permanent stabilization |
Types of Proteins: Globular vs Fibrous
🌱 Globular Proteins
- Generally soluble in water due to their compact, folded structure with hydrophilic groups on the outside.
- Have physiological roles such as enzymes, hormones, transport proteins (e.g., hemoglobin), and antibodies.
🌿 Fibrous Proteins
- Generally insoluble in water because of their long, fibrous, and repetitive structure with mostly hydrophobic amino acids exposed.
- Serve structural roles providing strength and support to cells and tissues (e.g., collagen in connective tissue, keratin in hair and nails).
| Protein Type | Solubility | Role |
|---|---|---|
| Globular | Soluble | Physiological functions (enzymes, transport, regulation) |
| Fibrous | Insoluble | Structural support (strength, protection) |
Structure of Haemoglobin: An Example of a Globular Protein
🌱 Basic Structure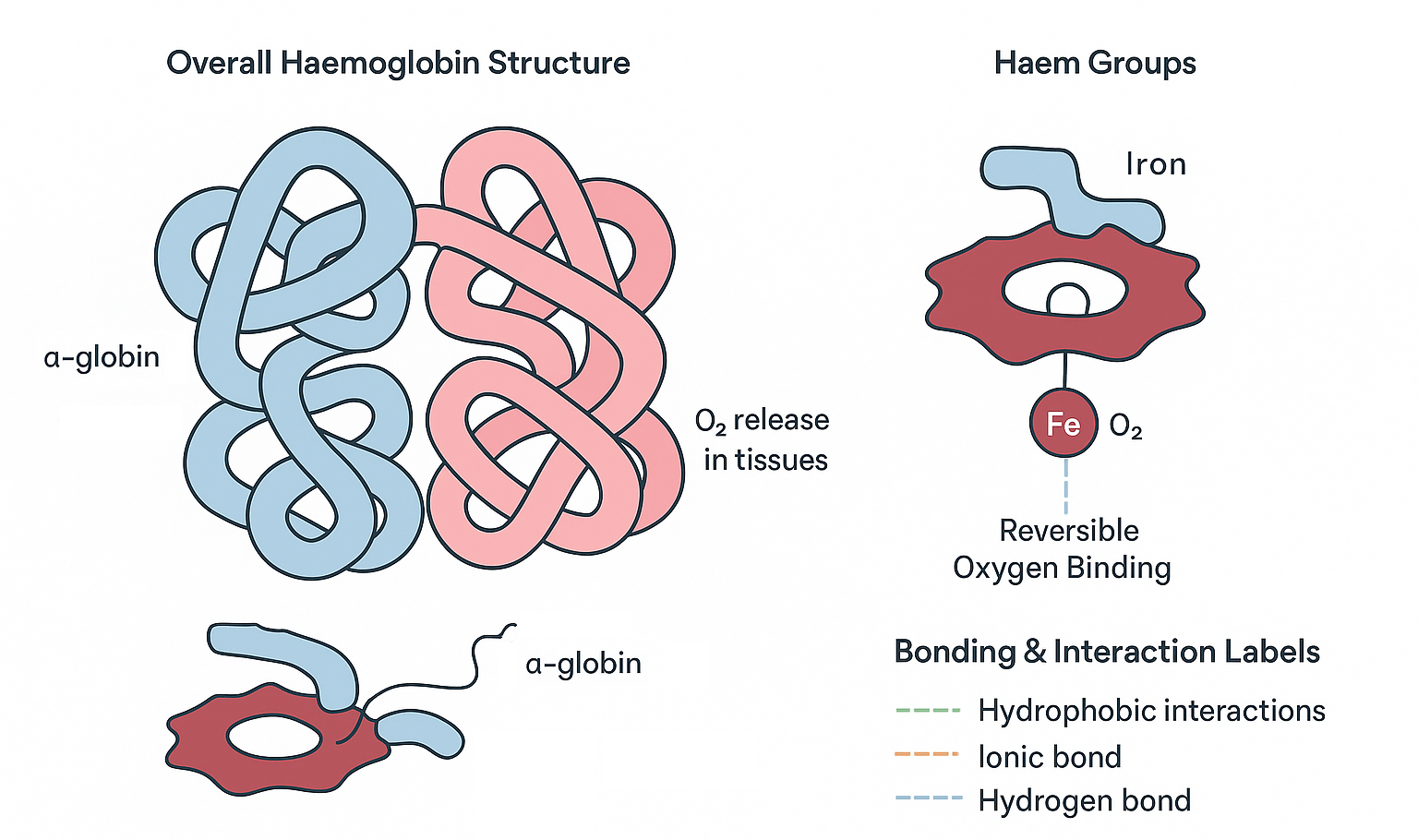
- Haemoglobin is a globular protein found in red blood cells.
- Its main function is to transport oxygen from the lungs to tissues.
🌿 Subunit Composition (Quaternary Structure)
- Haemoglobin’s quaternary structure is formed by four polypeptide chains:
- Two alpha (α) chains (α-globin)
- Two beta (β) chains (β-globin)
- Each chain is a globular polypeptide folded into a specific 3D shape.
🔬 Haem Group
- Each polypeptide chain contains a haem group, a prosthetic (non-protein) group.
- The haem group has an iron (Fe²⁺) ion at its center.
- This iron ion binds oxygen molecules reversibly.
🔗 Quaternary Structure Formation
- The four polypeptide chains are held together by non-covalent interactions:
- Hydrogen bonds
- Ionic bonds
- Hydrophobic interactions
- This arrangement allows haemoglobin to change shape during oxygen binding and release, facilitating efficient oxygen transport.
| Feature | Description |
|---|---|
| Protein Type | Globular |
| Number of Polypeptide Chains | Four (2 α-globin + 2 β-globin) |
| Prosthetic Group | Haem (contains Fe²⁺ ion) |
| Function | Oxygen transport |
| Quaternary Structure | Subunits held by hydrogen, ionic, and hydrophobic bonds |
Structure of Haemoglobin and Its Relation to Function
🌱 Key Structural Features of Haemoglobin
- Quaternary structure: Four polypeptide chains (2 α-globin + 2 β-globin) arranged to work cooperatively.
- Haem groups: Each chain contains one haem group with an iron (Fe²⁺) ion at the center.
- The protein’s globular shape allows it to be soluble in blood.
🔍 How Structure Supports Function
| Structural Feature | Functional Importance |
|---|---|
| Four subunits | Allows cooperative binding: binding of oxygen to one subunit increases affinity at others, enhancing oxygen uptake and release. |
| Haem group with Fe²⁺ ion | The iron ion binds oxygen reversibly, enabling haemoglobin to pick up oxygen in the lungs and release it in tissues. |
| Globular shape | Solubility in blood plasma allows efficient oxygen transport. |
| Flexible quaternary structure | Changes shape when oxygen binds (oxyhaemoglobin) and releases (deoxyhaemoglobin), optimizing oxygen delivery. |
🧠 Importance of Iron (Fe²⁺) in the Haem Group
- The Fe²⁺ ion is the active site for oxygen binding.
- It binds one oxygen molecule (O₂) per haem group, so each haemoglobin molecule can carry up to four oxygen molecules.
- The reversible binding is crucial for oxygen loading in lungs and unloading in tissues.
Haemoglobin’s structure – with multiple subunits and haem-bound iron – is perfectly adapted to efficient oxygen transport.
The iron ion in haem is essential for oxygen binding, making haemoglobin vital for respiration.
Structure of Collagen: An Example of a Fibrous Protein
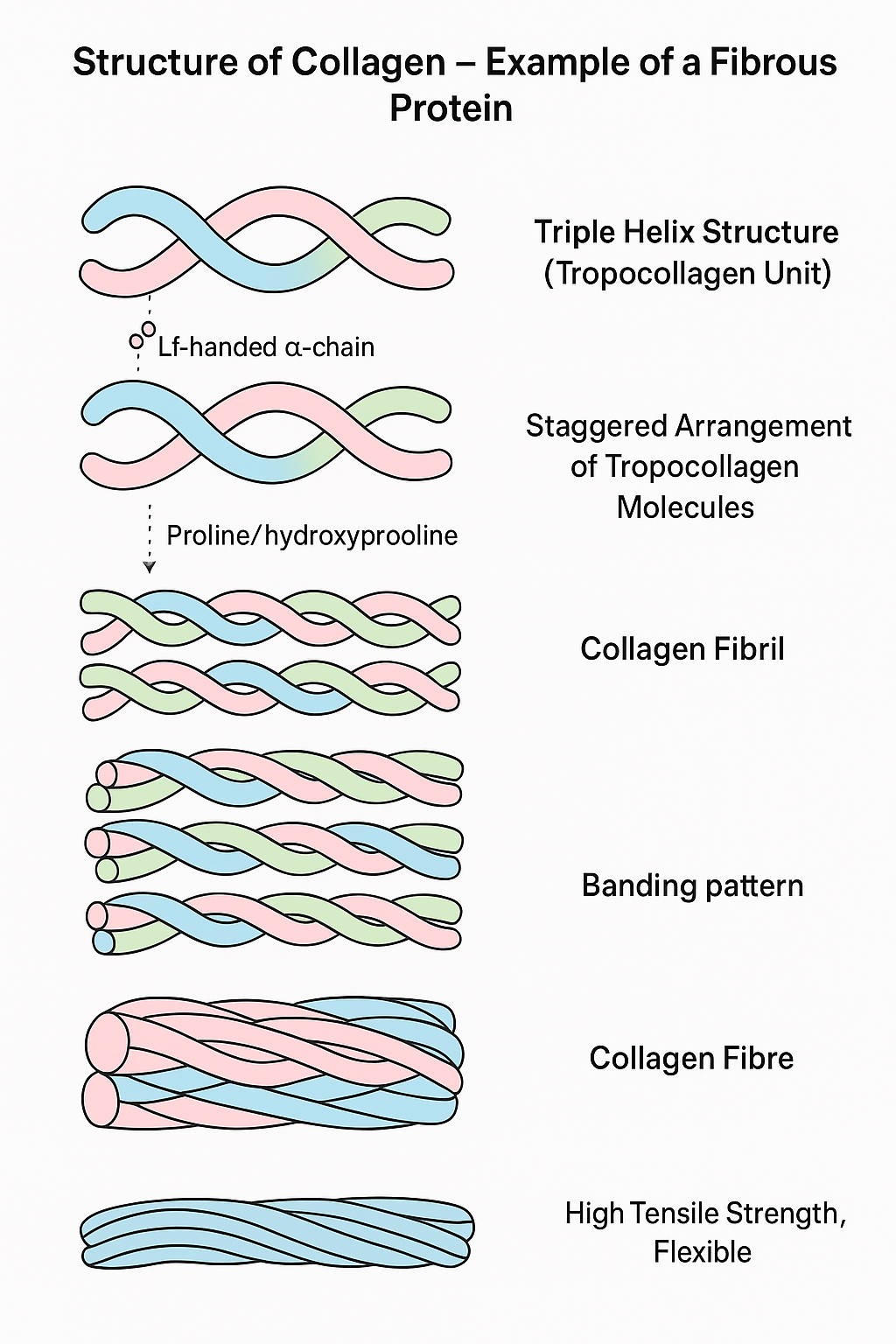
🌱 Basic Structure of a Collagen Molecule
- Collagen is a fibrous protein providing structural support in connective tissues.
- Its basic unit is a tropocollagen molecule made of three polypeptide chains (called α-chains).
- These three chains are left-handed helices twisted together into a right-handed triple helix.
- Each chain is rich in the amino acids glycine, proline, and hydroxyproline.
- Glycine appears at every third position, allowing the chains to pack tightly.
- Hydroxyproline helps stabilize the triple helix via hydrogen bonds.
🌿 Arrangement of Collagen Molecules
- Many tropocollagen molecules line up in a staggered, overlapping manner to form collagen fibrils.
- Fibrils are stabilized by cross-links (covalent bonds) between lysine residues in adjacent molecules, increasing tensile strength.
- Multiple fibrils bundle together to form collagen fibres, which are visible under a microscope.
- This hierarchical structure provides high tensile strength and flexibility.
| Level | Description |
|---|---|
| Tropocollagen | Triple helix of 3 α-polypeptide chains |
| Fibrils | Staggered, cross-linked tropocollagen molecules |
| Fibres | Bundles of collagen fibrils |
| Function | Provides strength and support in connective tissue |
Relationship Between Structure and Function of Collagen Molecules and Fibres
🌱 Structural Features of Collagen
| Structure Level | Key Features |
|---|---|
| Collagen Molecules | Triple helix formed by three α-chains, rich in glycine, proline, and hydroxyproline. Tight packing and hydrogen bonding provide stability. |
| Collagen Fibrils | Staggered arrangement of molecules with covalent cross-links between lysine residues increases tensile strength. |
| Collagen Fibres | Bundles of fibrils form thick, strong fibres with flexibility and durability. |
🔍 How Structure Relates to Function
| Structural Feature | Functional Benefit |
|---|---|
| Triple helix structure | Provides high tensile strength and resistance to stretching forces. |
| High glycine content | Allows tight packing of chains for a compact, strong structure. |
| Hydrogen bonds and cross-links | Stabilize the molecule and fibrils, enhancing durability and mechanical strength. |
| Fibril staggered arrangement | Distributes mechanical stress evenly, preventing damage. |
| Bundle formation into fibres | Produces tough, flexible connective tissue structures (e.g., tendons, ligaments, skin). |
The hierarchical organisation from molecules to fibres allows collagen to resist pulling forces while maintaining flexibility, making it essential for structural support in animals.
