CIE AS/A Level Biology -2.4 Water- Study Notes- New Syllabus
CIE AS/A Level Biology -2.4 Water- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -2.4 Water- Study Notes- New Syllabus
Key Concepts:
- explain how hydrogen bonding occurs between water molecules and relate the properties of water to its roles in living organisms, limited to solvent action, high specific heat capacity and latent heat of vaporisation
Hydrogen Bonding in Water and Its Biological Importance
🌱 How Hydrogen Bonding Occurs Between Water Molecules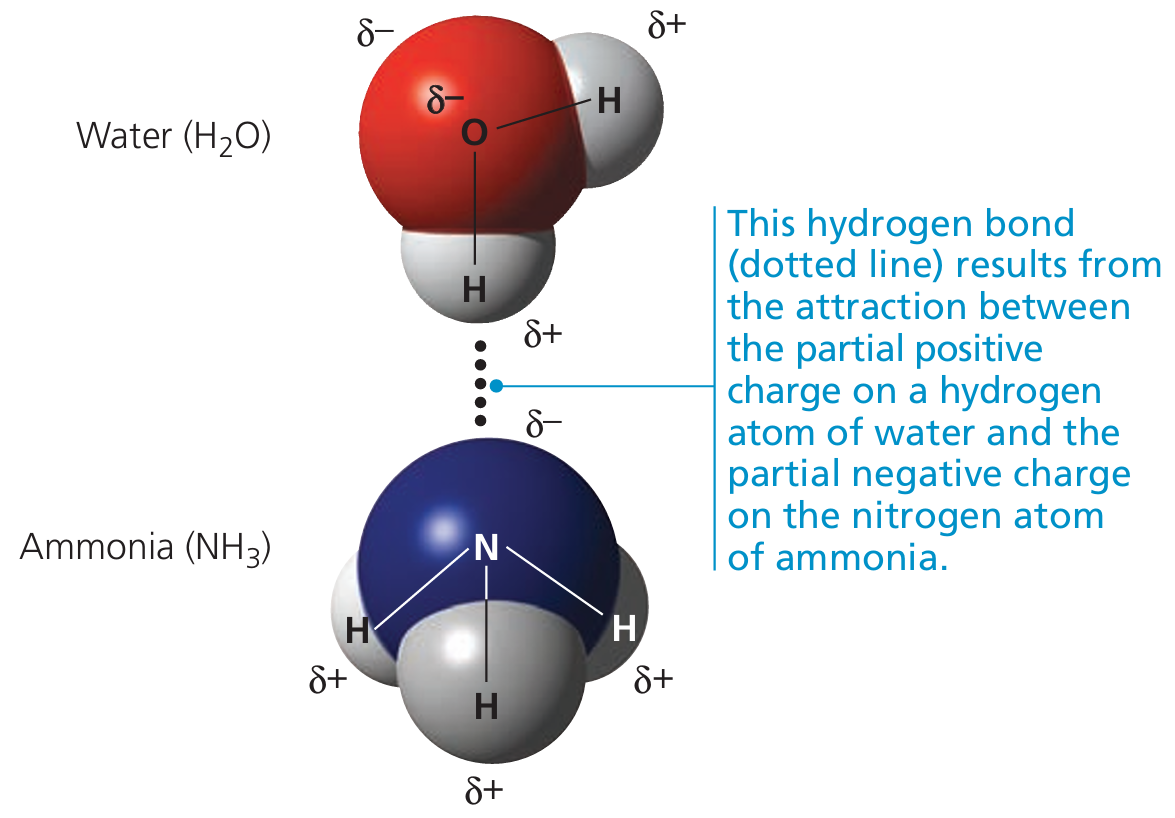
- A water molecule (H₂O) has a polar structure:
- Oxygen is more electronegative and carries a partial negative charge (δ⁻).
- Hydrogen atoms carry partial positive charges (δ⁺).
- Hydrogen bonds form when the δ⁺ hydrogen of one water molecule is attracted to the δ⁻ oxygen of a neighboring water molecule.
- These are weak intermolecular forces, but collectively strong due to many bonds forming between molecules.
🌿 Properties of Water Related to Hydrogen Bonding
| Property | Description | Role in Living Organisms |
|---|---|---|
| Solvent Action | Water dissolves many polar and ionic substances because its polarity allows it to surround and separate solute molecules/ions. | Enables transport of nutrients, gases, and wastes in blood and cytoplasm. |
| High Specific Heat Capacity | Water absorbs a lot of heat before its temperature rises significantly due to energy needed to break hydrogen bonds. | Helps maintain stable internal temperatures in organisms and environments. |
| High Latent Heat of Vaporisation | Water requires significant energy to evaporate, breaking many hydrogen bonds. | Allows organisms to cool efficiently through sweating or transpiration without losing too much water. |
🧠 Key Points
- Hydrogen bonding gives water unique properties essential for life.
- Water’s ability as a universal solvent supports biochemical reactions and transport.
- Its thermal properties stabilize temperature and support temperature regulation in organisms.
