CIE AS/A Level Biology -3.1 Mode of action of enzymes- Study Notes- New Syllabus
CIE AS/A Level Biology -3.1 Mode of action of enzymes- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -3.1 Mode of action of enzymes- Study Notes- New Syllabus
Key Concepts:
- state that enzymes are globular proteins that catalyse reactions inside cells (intracellular enzymes) or are secreted to catalyse reactions outside cells (extracellular enzymes)
- explain the mode of action of enzymes in terms of an active site, enzyme–substrate complex, lowering of activation energy and enzyme specificity, including the lock-and-key hypothesis and the induced-fit hypothesis
- investigate the progress of enzyme-catalysed reactions by measuring rates of formation of products using catalase and rates of disappearance of substrate using amylase
- outline the use of a colorimeter for measuring the progress of enzyme-catalysed reactions that involve colour changes
Enzymes: Nature and Function
🌱 What Are Enzymes?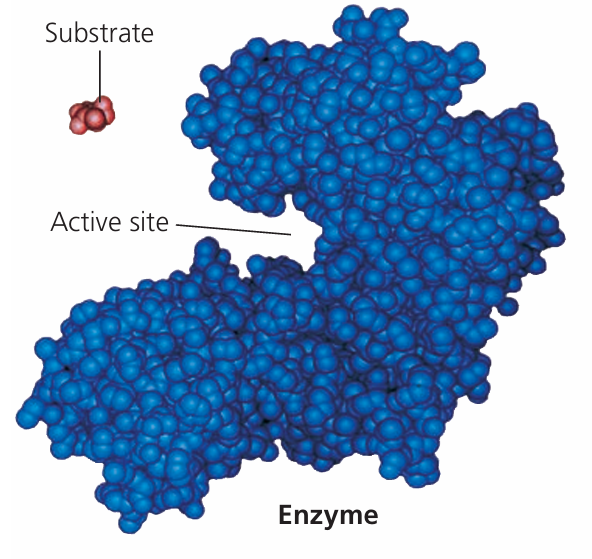
- Enzymes are globular proteins that act as biological catalysts.
- They speed up chemical reactions without being consumed.
🌿 Types of Enzymes Based on Location
| Enzyme Type | Location | Function |
|---|---|---|
| Intracellular enzymes | Inside cells | Catalyse reactions within cells (e.g., DNA polymerase in replication). |
| Extracellular enzymes | Secreted outside cells | Catalyse reactions outside cells (e.g., amylase in saliva breaking down starch). |
🧠 Key Points
- Enzymes lower the activation energy of reactions, increasing reaction rates.
- Their globular structure allows specific substrate binding.
- Enzymes are vital for metabolism and other cellular processes.
Mode of Action of Enzymes
🌱 Active Site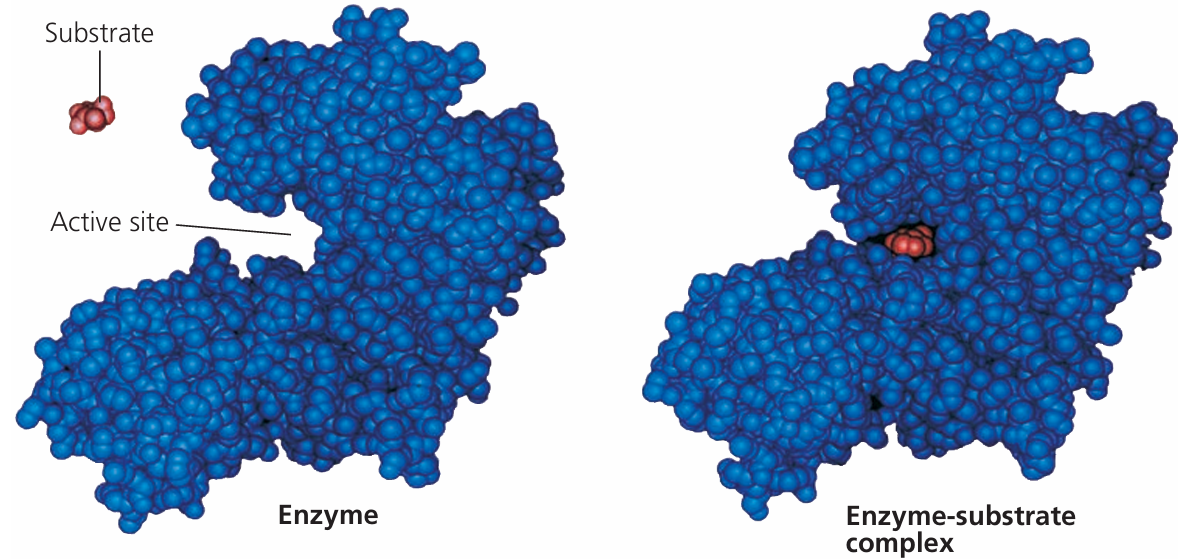
- The active site is a specific region on the enzyme where the substrate binds.
- It has a unique shape and chemical environment complementary to the substrate.
🔍 Enzyme–Substrate Complex
- When the substrate binds to the active site, an enzyme–substrate complex forms.
- This complex stabilizes the transition state, facilitating the conversion to products.
⚡ Lowering Activation Energy
- Enzymes lower the activation energy required for a reaction, making it easier and faster to occur.
- They do this by:
- Bringing substrates closer in the correct orientation.
- Stressing particular chemical bonds in substrates.
- Providing an optimal microenvironment.
🎯 Enzyme Specificity
- Enzymes are highly specific-each enzyme typically binds only one substrate or a group of closely related substrates.
🧠 Hypotheses Explaining Enzyme Specificity
| Hypothesis | Description |
|---|---|
| Lock-and-Key | The substrate fits exactly into the active site like a key fits a specific lock. |
| Induced-Fit | The active site changes shape slightly to fit the substrate after binding, improving interaction. |
📌 Summary
| Concept | Explanation |
|---|---|
| Active Site | Specific region for substrate binding |
| Enzyme–Substrate Complex | Temporary complex stabilizing the reaction |
| Activation Energy | Energy barrier lowered by enzyme to speed up reaction |
| Specificity | Enzymes bind specific substrates (lock-and-key or induced fit) |
Investigating Enzyme-Catalysed Reactions: Measuring Reaction Rates
🌱 Overview
- Enzyme activity can be studied by measuring either:
- Rate of product formation (how fast the product appears), or
- Rate of substrate disappearance (how fast the substrate is used up).
🔬 Example 1: Catalase (Measuring Rate of Product Formation)
Catalase catalyses the breakdown of hydrogen peroxide (H₂O₂) into water and oxygen:
2H₂O₂ → 2H₂O + O₂
Method:
- Measure the volume or rate of oxygen gas produced over time using a gas syringe or displacement of water.
- The faster oxygen is produced, the higher the enzyme activity.
Key point: Oxygen production is a direct measure of catalase activity.
🔍 Example 2: Amylase (Measuring Rate of Substrate Disappearance)
Amylase breaks down starch into maltose and glucose.
Method:
- Mix amylase with a starch solution and take samples at regular intervals.
- Use iodine test on samples: iodine stains starch blue-black; as starch disappears, the colour fades.
- Measure the time taken for starch to disappear or quantify colour change using a colorimeter.
Key point: Disappearance of starch indicates the rate of amylase activity.
📊 Summary Table
| Enzyme | Substrate | Measured Quantity | Method |
|---|---|---|---|
| Catalase | Hydrogen peroxide | Rate of oxygen gas formation | Gas syringe or water displacement method |
| Amylase | Starch | Rate of starch disappearance | Iodine test with timed sampling |
🧠 Important Considerations
- Keep conditions (temperature, pH, substrate concentration) constant to ensure fair comparison.
- Repeat trials for accuracy and calculate mean rates.
- Use appropriate controls (e.g., no enzyme) to confirm results.
Using a Colorimeter to Measure Enzyme-Catalysed Reactions Involving Colour Changes
🌱 What is a Colorimeter?
- A colorimeter is an instrument that measures the intensity of colour in a solution.
- It quantifies how much light of a specific wavelength is absorbed by the coloured solution.
🌿 Why Use a Colorimeter in Enzyme Experiments?
- Many enzyme reactions cause a change in colour of the reaction mixture (e.g., starch breakdown detected by iodine).
- The colorimeter measures this colour change objectively, allowing accurate tracking of reaction progress.
🔍 How to Use a Colorimeter
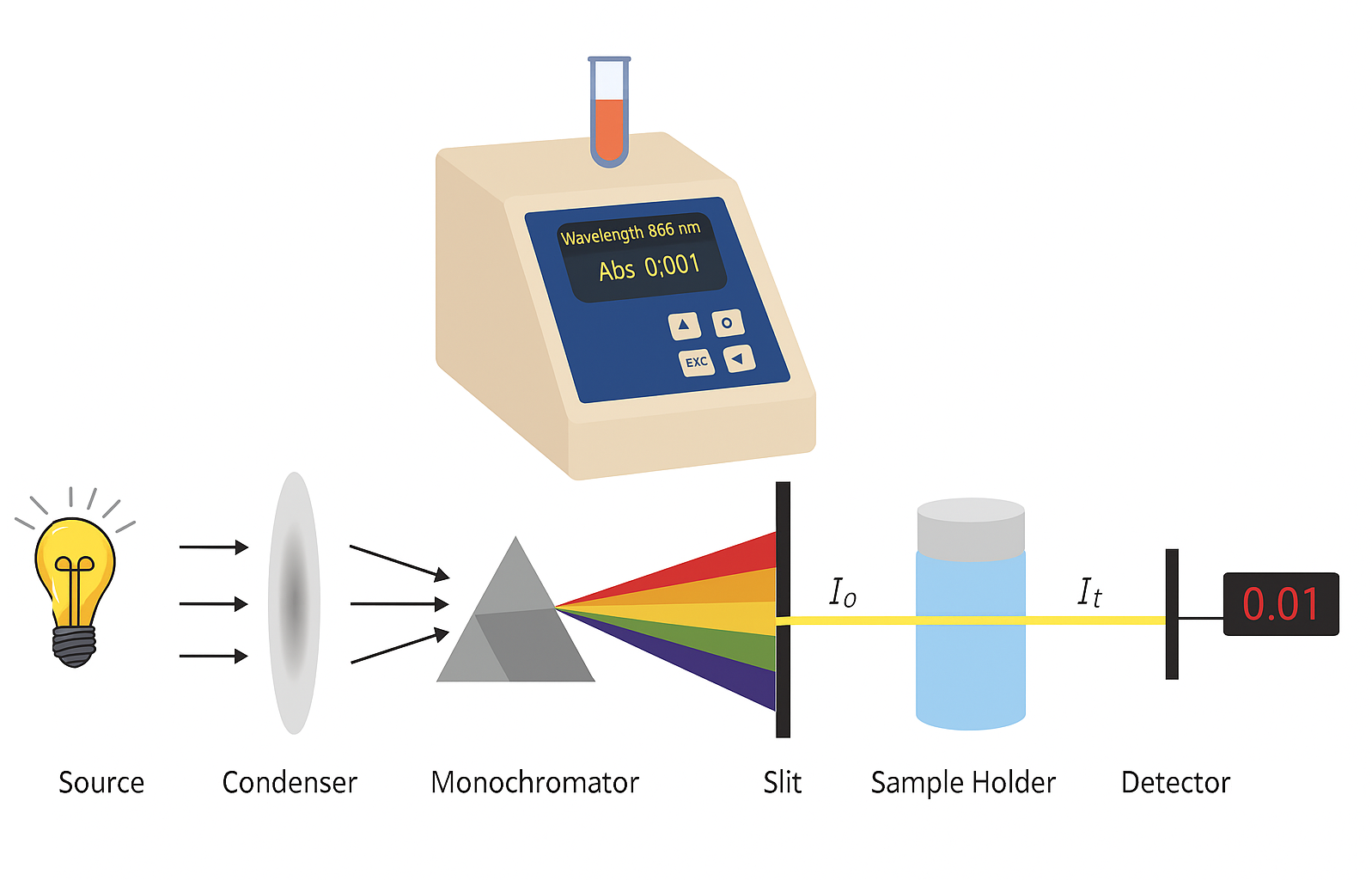
- Prepare samples of the reaction mixture at different time intervals during the enzyme-catalysed reaction.
- Select the appropriate wavelength that corresponds to the colour of the substrate or product (e.g., blue-black for starch with iodine).
- Place the sample in a cuvette and insert it into the colorimeter.
- The colorimeter passes light through the sample and measures the absorbance (or transmission) of light.
- Record the absorbance values at each time point to monitor changes.
- Plot absorbance against time to determine the rate of reaction.
📌 Example
- In an amylase reaction, starch is broken down, causing the blue-black colour (from iodine) to fade.
- As starch concentration decreases, absorbance at the wavelength for the blue-black colour decreases.
- The rate at which absorbance drops reflects the rate of starch breakdown by amylase.
🧠 Key Points
- Colorimetry provides a quantitative, objective way to measure enzyme activity.
- It is sensitive and can detect even small changes in substrate or product concentration.
- Ensure consistent cuvette use and calibration with blanks (e.g., iodine solution without starch) for accuracy.
