CIE AS/A Level Biology -3.2 Factors that affect enzyme action- Study Notes- New Syllabus
CIE AS/A Level Biology -3.2 Factors that affect enzyme action- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -3.2 Factors that affect enzyme action- Study Notes- New Syllabus
Key Concepts:
- investigate and explain the effects of the following factors on the rate of enzyme-catalysed reactions:
• temperature
• pH (using buffer solutions)
• enzyme concentration
• substrate concentration
• inhibitor concentration - explain that the maximum rate of reaction (Vmax) is used to derive the Michaelis–Menten constant (Km), which is used to compare the affinity of different enzymes for their substrates
- explain the effects of reversible inhibitors, both competitive and non-competitive, on enzyme activity
- investigate the difference in activity between an enzyme immobilised in alginate and the same enzyme free in solution, and state the advantages of using immobilised enzymes
Factors Affecting the Rate of Enzyme-Catalysed Reactions
🌡️ 1. Temperature
- Effect:
- Increasing temperature generally increases reaction rate by providing more kinetic energy, so more enzyme-substrate collisions occur.
- Optimal temperature: Each enzyme has a temperature where it works best.
- Above this, the enzyme denatures (loses shape), causing a sharp decrease in activity.
- Below optimal temperature, reactions proceed slowly.
- Explanation: Heat disrupts hydrogen bonds and other interactions maintaining enzyme shape.
⚖️ 2. pH (Using Buffer Solutions)
- Effect:
- Each enzyme has an optimal pH where activity is highest.
- Deviations from optimal pH cause changes in enzyme structure and alter the charge of amino acids, especially at the active site, reducing activity.
- Extreme pH values lead to denaturation.
- Explanation: Buffers maintain constant pH during experiments, ensuring reliable results.
🧫 3. Enzyme Concentration
- Effect:
- Increasing enzyme concentration generally increases reaction rate, provided there is excess substrate.
- Rate increases proportionally to enzyme concentration.
- If substrate becomes limiting, rate plateaus.
🍬 4. Substrate Concentration
- Effect:
- Increasing substrate concentration increases the rate, as more substrate molecules collide with enzymes.
- Rate rises until all enzyme active sites are occupied (saturation point); then it plateaus.
- After saturation, adding more substrate does not increase rate.
🚫 5. Inhibitor Concentration
- Effect: Inhibitors decrease enzyme activity by binding to the enzyme and reducing its ability to catalyse reactions.
- Increasing inhibitor concentration generally reduces reaction rate further.
- Types:
- Competitive inhibitors compete with substrate for the active site.
- Non-competitive inhibitors bind elsewhere, changing enzyme shape.
📊 Summary Table
| Factor | Effect on Rate | Explanation |
|---|---|---|
| Temperature | Increases rate to optimum; denaturation after | Kinetic energy and enzyme stability |
| pH | Optimal pH for max rate; denaturation outside range | Charge and shape of enzyme |
| Enzyme concentration | Rate increases with enzyme, plateaus if substrate limited | Number of active sites available |
| Substrate concentration | Rate increases with substrate, plateaus at saturation | Active site availability |
| Inhibitor concentration | Rate decreases as inhibitors block enzyme action | Inhibitor binding to enzyme |
🧠 Key Points
Enzymes are sensitive to environmental conditions.
Understanding these factors is crucial for controlling and optimizing enzyme activity in biological and industrial processes.
Michaelis-Menten Constant (Km) and Maximum Reaction Rate (Vmax)
🌱 Maximum Rate of Reaction (Vmax)
- Vmax is the maximum rate at which an enzyme can catalyse a reaction when the substrate concentration is very high.
- At Vmax, all enzyme active sites are saturated with substrate, so increasing substrate further does not increase the rate.
🌿 Michaelis-Menten Constant (Km)
- Km is the substrate concentration at which the reaction rate is half of Vmax.
- It is a measure of the affinity of an enzyme for its substrate.
- Low Km: High affinity (enzyme reaches half-maximal rate at low substrate concentration).
- High Km: Low affinity (requires higher substrate concentration to reach half-maximal rate).
🔍 How Vmax and Km are Used
- By measuring reaction rates at different substrate concentrations, a graph of rate vs substrate concentration is plotted.
- Vmax is determined from the plateau of this graph.
- Km is found at the substrate concentration corresponding to half of Vmax.
- Comparing Km values for different enzymes or substrates helps determine which enzyme binds its substrate more tightly.
📌 Summary
| Term | Definition | Interpretation |
|---|---|---|
| Vmax | Maximum reaction rate at saturating substrate | Indicates catalytic capacity |
| Km | Substrate concentration at ½ Vmax | Indicates enzyme-substrate affinity |
🧠 Key Point
Km provides insight into enzyme efficiency and substrate binding, essential for understanding enzyme kinetics and drug design.
Effects of Reversible Inhibitors on Enzyme Activity
🌱 Reversible Inhibitors
- These inhibitors bind temporarily to enzymes and can be removed, allowing the enzyme to regain activity.
- Two main types: competitive and non-competitive inhibitors.
🌿 1. Competitive Inhibitors
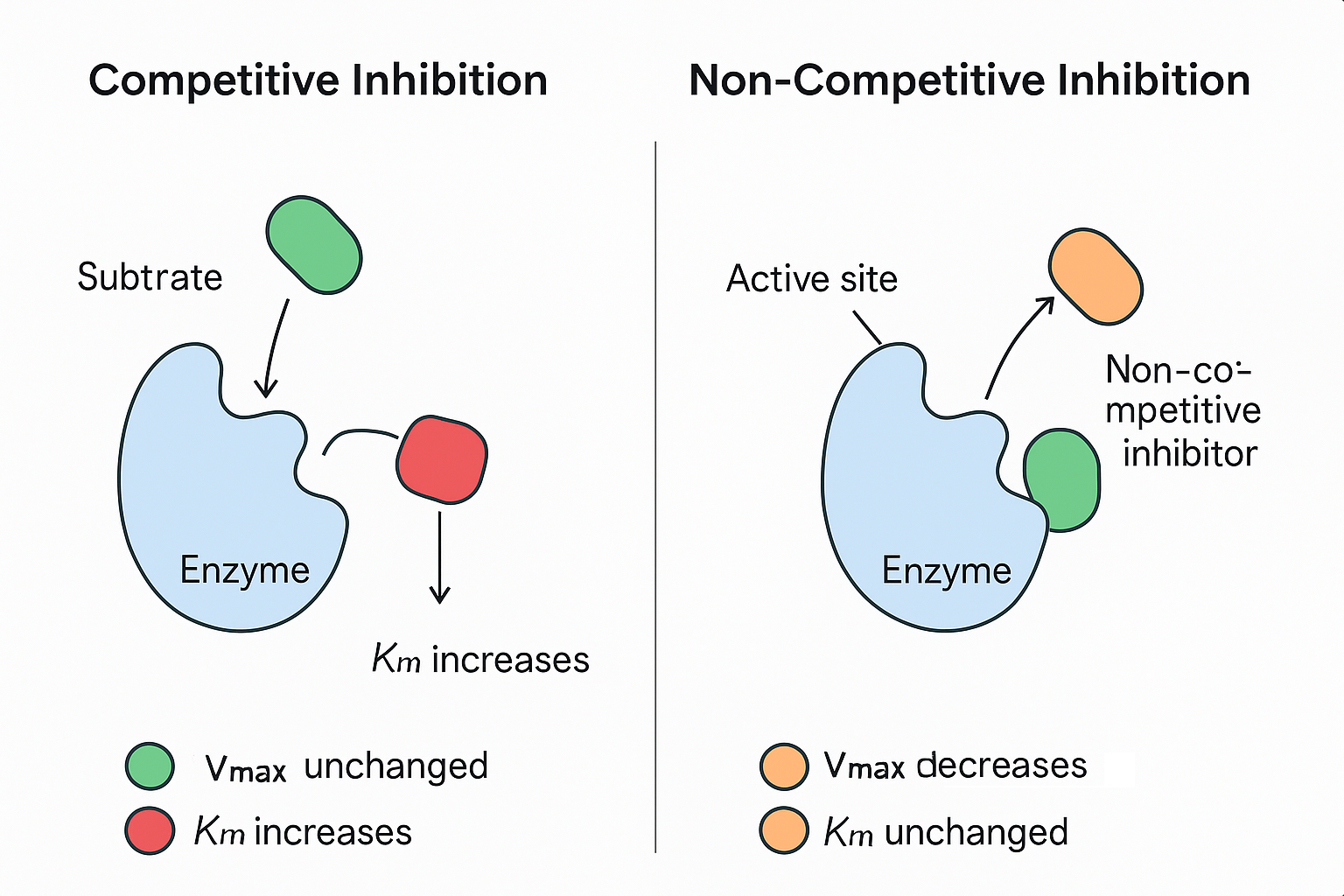
- Mechanism:
- Compete with the substrate for binding to the active site of the enzyme.
- Their shape is similar to the substrate, allowing them to block substrate binding.
- Effect on Enzyme Activity:
- Increase Km (more substrate needed to reach half Vmax) because substrate and inhibitor compete for the same site.
- No change in Vmax, as high substrate concentration can outcompete the inhibitor.
🔬 2. Non-Competitive Inhibitors
- Mechanism:
- Bind to a different site (allosteric site) on the enzyme, not the active site.
- Binding changes the enzyme’s shape, reducing its activity regardless of substrate concentration.
- Effect on Enzyme Activity:
- Decrease Vmax (maximum rate is lowered) because some enzyme molecules become inactive.
- Km remains unchanged, as substrate binding is not directly affected.
📊 Comparison Table
| Feature | Competitive Inhibition | Non-Competitive Inhibition |
|---|---|---|
| Binding Site | Active site | Allosteric site |
| Effect on Km | Increases (lower substrate affinity) | No change |
| Effect on Vmax | No change | Decreases |
| Overcome by | Increasing substrate concentration | Cannot be overcome by substrate increase |
🧠 Key Points
Competitive inhibitors slow enzyme activity by blocking substrate access.
Non-competitive inhibitors alter enzyme function by changing its shape.
Both regulate enzyme activity but via different mechanisms.
Investigating Enzyme Activity: Immobilised vs. Free Enzymes
🌱 Enzyme Immobilisation
- Immobilised enzymes are enzymes physically confined or attached to a solid support (e.g., alginate beads), restricting their movement.
- Free enzymes float freely in solution and are not fixed.
🔍 Comparing Activity
| Feature | Free Enzyme | Immobilised Enzyme |
|---|---|---|
| Reaction rate | Usually faster initially due to free movement and easy substrate access. | May be slower initially because substrate must diffuse into the matrix. |
| Reusability | Cannot be reused easily; lost after reaction. | Can be reused multiple times, reducing costs. |
| Stability | More sensitive to changes in temperature and pH. | Often more stable under varying conditions. |
| Control | Harder to separate enzyme from product. | Easier to separate enzyme from reaction mixture, preventing contamination. |
🌿 Advantages of Using Immobilised Enzymes
- Reusability: Can be recovered and reused for many cycles.
- Increased Stability: Enhanced resistance to pH and temperature changes.
- Product Purity: Easier separation from reaction products, reducing downstream processing.
- Continuous Processes: Suitable for continuous industrial processes, improving efficiency.
- Controlled Reaction: Reduced enzyme contamination in products.
Immobilised enzymes may show slightly lower reaction rates due to diffusion limitations but offer significant economic and practical advantages in industrial applications.
Their stability and reusability make them ideal for repeated or continuous use.
