CIE AS/A Level Biology -4.2 Movement into and out of cells- Study Notes- New Syllabus
CIE AS/A Level Biology -4.2 Movement into and out of cells- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -4.2 Movement into and out of cells- Study Notes- New Syllabus
Key Concepts:
- describe and explain the processes of simple diffusion, facilitated diffusion, osmosis, active transport, endocytosis and exocytosis
- investigate simple diffusion and osmosis using plant tissue and non-living materials, including dialysis (Visking) tubing and agar
- illustrate the principle that surface area to volume ratios decrease with increasing size by calculating surface areas and volumes of simple 3-D shapes (as shown in the Mathematical requirements)
- investigate the effect of changing surface area to volume ratio on diffusion using agar blocks of different sizes
- investigate the effects of immersing plant tissues in solutions of different water potentials, using the results to estimate the water potential of the tissues
- explain the movement of water between cells and solutions in terms of water potential and explain the different effects of the movement of water on plant cells and animal cells (knowledge of solute potential and pressure potential is not expected)
Membrane Transport Processes
🌬 1. Simple Diffusion
- Definition: Passive movement of molecules from a region of higher concentration to lower concentration until equilibrium is reached.
- Energy requirement: No energy (passive process).
- Molecules moved: Small, non-polar molecules (O₂, CO₂, lipid-soluble substances).
- Mechanism: Molecules move directly through the phospholipid bilayer.
- Rate affected by: Concentration gradient, temperature, surface area, and membrane thickness.
🚇 2. Facilitated Diffusion
- Definition: Passive transport of molecules across a membrane via specific transport proteins.
- Energy requirement: No energy (passive).
- Molecules moved: Larger or polar molecules (e.g., glucose, amino acids) and ions.
- Types of proteins involved:
- Channel proteins – form pores for ions/molecules to diffuse through.
- Carrier proteins – change shape to transport substances.
- Specificity: Each protein is specific to one type of molecule or ion.
💧 3. Osmosis
- Definition: The net movement of water molecules across a partially permeable membrane from a region of higher water potential to lower water potential.
- Passive process: No energy required.
- Key terms:
- Water potential (Ψ) – tendency of water to move; pure water Ψ = 0 kPa.
- Lower Ψ = hypertonic; higher Ψ = hypotonic.
- Importance: Maintains cell turgor in plants, regulates water balance in animals.
⚡ 4. Active Transport
- Definition: Movement of molecules against the concentration gradient using energy from ATP.
- Molecules moved: Ions (Na⁺, K⁺), glucose, amino acids.
- Protein involved: Carrier proteins (pumps).
- Energy source: ATP provides energy to change carrier protein shape.
- Example: Sodium–potassium pump in nerve cells.
📦 5. Endocytosis
- Definition: Process where the cell membrane engulfs materials into the cell, forming a vesicle.
- Energy requirement: Active process (requires ATP).
- Types:
- Phagocytosis – “cell eating” (engulfing large particles, e.g., bacteria by white blood cells).
- Pinocytosis – “cell drinking” (engulfing small molecules and fluids).
- Mechanism: Membrane folds inwards, surrounds material, forms vesicle inside cytoplasm.
📤 6. Exocytosis
- Definition: Process where materials are exported out of the cell via vesicles.
- Energy requirement: Active process (requires ATP).
- Mechanism: Vesicle fuses with cell membrane and contents are released outside the cell.
- Example: Secretion of neurotransmitters, enzymes, and hormones.
| Process | Passive/Active | Uses ATP? | Direction of Movement | Example |
|---|---|---|---|---|
| Simple diffusion | Passive | ✘ | High → Low conc. | O₂ entering cell |
| Facilitated diffusion | Passive | ✘ | High → Low conc. | Glucose via carrier protein |
| Osmosis | Passive | ✘ | High → Low Ψ | Water entering plant roots |
| Active transport | Active | ✔ | Low → High conc. | Na⁺/K⁺ pump |
| Endocytosis | Active | ✔ | Into cell | Phagocytosis of bacteria |
| Exocytosis | Active | ✔ | Out of cell | Release of insulin |
Cells transport materials by passive processes (diffusion, facilitated diffusion, osmosis) that require no ATP, and active processes (active transport, endocytosis, exocytosis) that use ATP. The method depends on molecule size, polarity, concentration gradient, and cell requirements.
Investigating Simple Diffusion & Osmosis
🌱 1. Key Concepts
- Diffusion: Movement of particles from a region of higher concentration to lower concentration due to random molecular motion.
- Osmosis: Movement of water molecules through a selectively permeable membrane from a region of higher water potential to a region of lower water potential.
🧪 2. Osmosis Using Plant Tissue (Potato or Beetroot)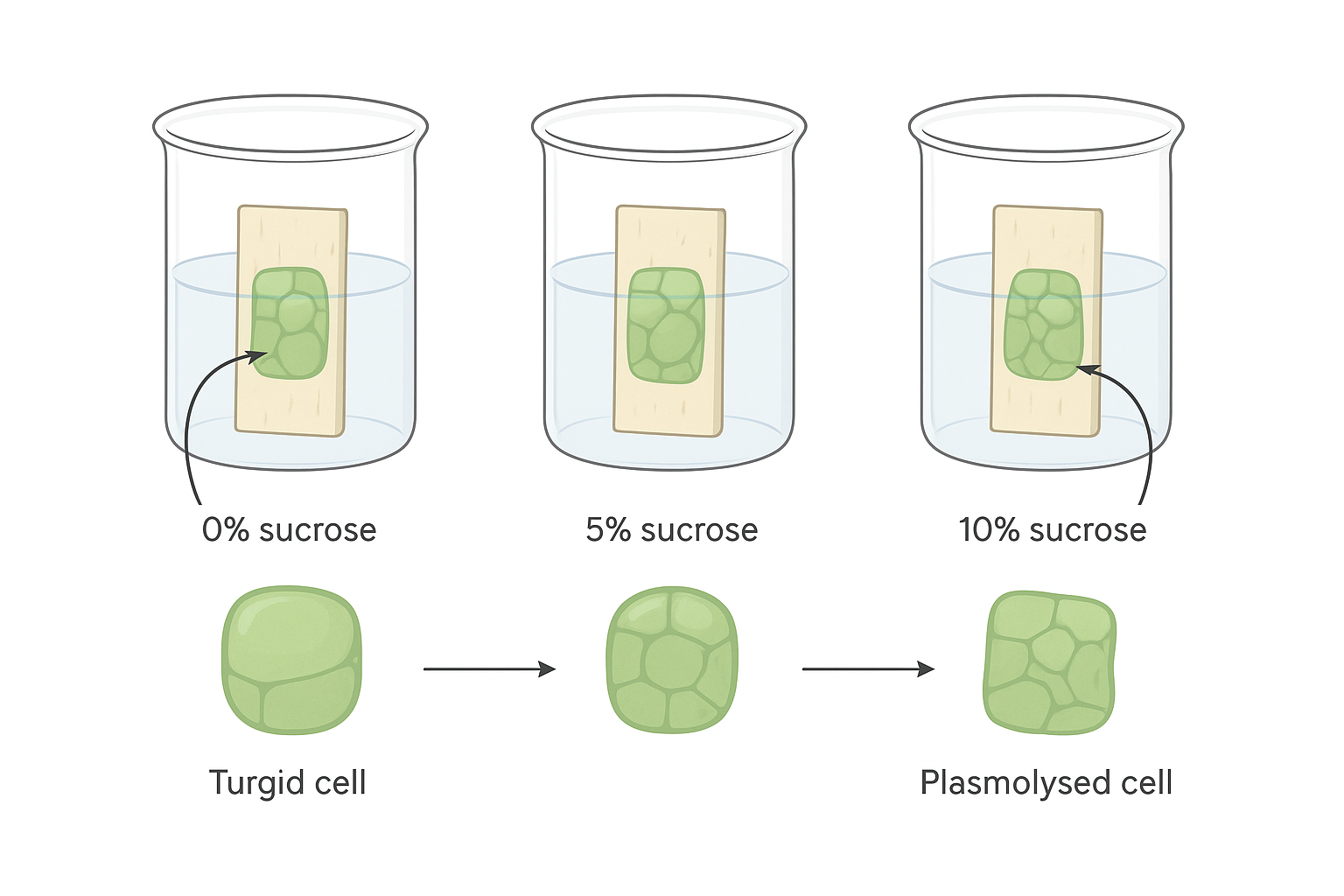
- Aim: Observe the effect of different solute concentrations on plant tissue.
- Materials: Potato/beetroot strips or cylinders, sucrose/salt solutions of various concentrations, distilled water, beakers, ruler, balance.
- Method:
- Cut equal-sized plant tissue samples.
- Record their initial length and/or mass.
- Place samples in different concentrations of solution (e.g., 0%, 5%, 10% sucrose).
- Leave for 30–60 minutes.
- Remove, blot dry, re-measure length/mass.
- Compare changes to determine water movement.
- Expected Results:
- Dilute solution: Tissue gains mass (water enters cells).
- Concentrated solution: Tissue loses mass (water leaves cells).
🧪 3. Osmosis Using Non-Living Material (Dialysis/Visking Tubing)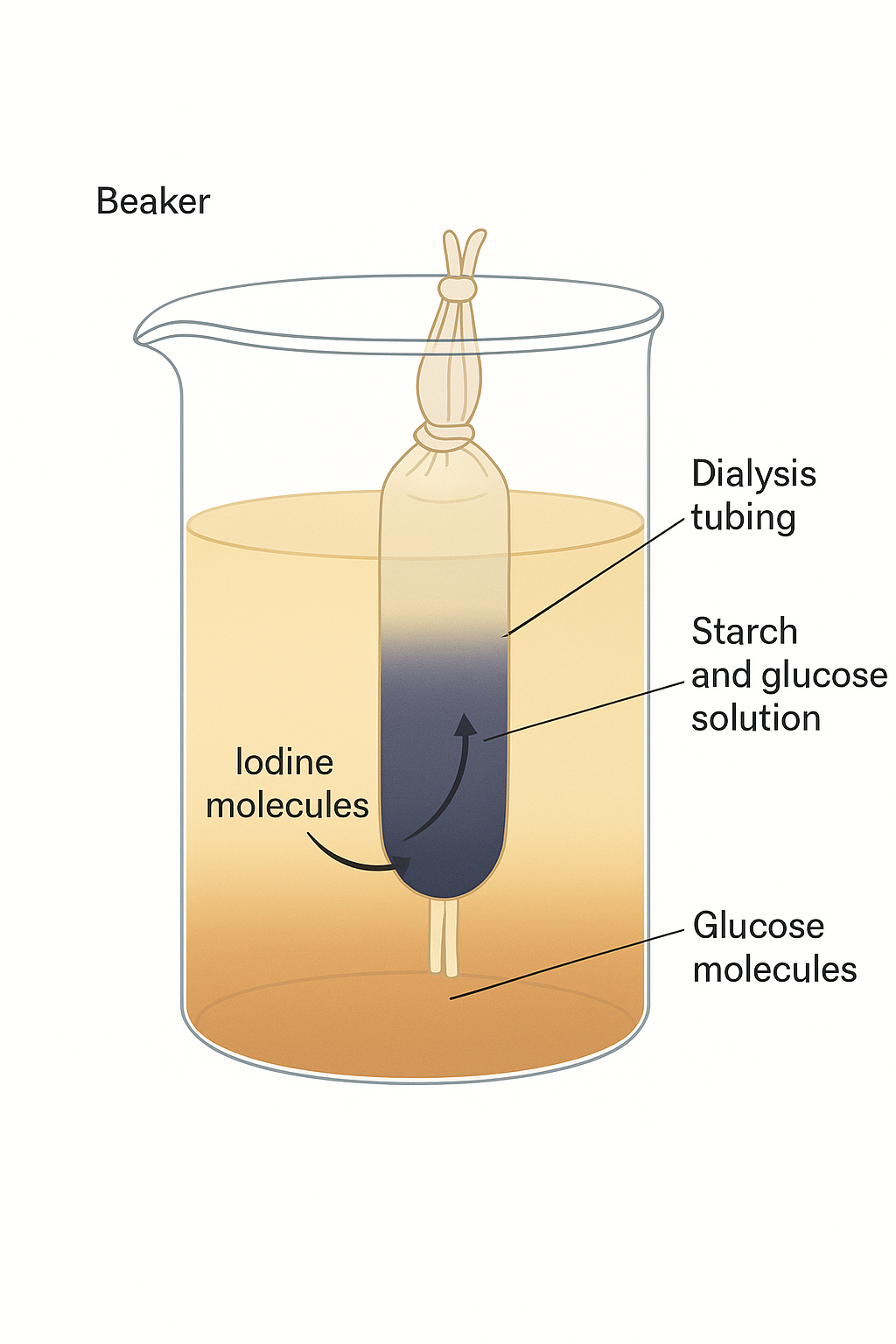
- Aim: Demonstrate selective permeability.
- Materials: Dialysis tubing, starch solution, glucose solution, water, iodine solution, Benedict’s reagent.
- Method:
- Fill tubing with starch + glucose solution, tie ends.
- Place in beaker of water + iodine.
- Leave for ~30 minutes.
- Test outside solution for glucose using Benedict’s test.
- Expected Results:
- Iodine enters tubing → starch turns blue-black.
- Glucose leaves tubing → detected outside using Benedict’s test.
🧪 4. Diffusion Using Agar Cubes
- Aim: Investigate effect of surface area to volume ratio on diffusion.
- Materials: Agar jelly with indicator (e.g., phenolphthalein), sodium hydroxide, hydrochloric acid, scalpel, ruler.
- Method:
- Cut agar into cubes of different sizes.
- Place cubes in dilute acid.
- Measure time for colour to disappear completely.
- Expected Results: Smaller cubes lose colour faster → higher SA:V ratio = faster diffusion.
📊 5. Summary Table
| Investigation | Material Used | Main Principle | Expected Outcome |
|---|---|---|---|
| Osmosis in plant tissue | Potato/Beetroot | Water movement depends on solution concentration | Mass/length changes |
| Osmosis in dialysis tubing | Visking tubing | Selective permeability | Small molecules pass, large molecules do not |
| Diffusion in agar cubes | Agar + indicator | Rate depends on SA:V ratio | Smaller cubes change colour faster |
Diffusion = particles move from high to low concentration.
Osmosis = water moves through a selectively permeable membrane.
Plant tissue shows osmotic gain/loss of mass.
Dialysis tubing mimics selective permeability of cell membranes.
Smaller cells or cubes diffuse substances faster due to higher surface area-to-volume ratio.
Surface Area to Volume Ratio (SA:V) and Cell Size
🌱 1. Principle
- Surface area (SA): Total area covering the outside of an object.
- Volume (V): Amount of space inside the object.
- SA:V ratio: Surface Area ÷ Volume
- As an object gets larger, SA:V ratio decreases – meaning less surface area is available for exchange of substances per unit volume.
🔍 2. Why It Matters for Cells
- Small cells have a large SA:V → faster exchange of gases, nutrients, and waste.
- Large cells have a small SA:V → slower exchange → cells must divide or adapt to survive.
📊 3. Calculating SA:V for a Cube
- Formula: SA = 6 × (side length)², V = (side length)³
| Side Length (cm) | Surface Area (cm²) | Volume (cm³) | SA:V Ratio |
|---|---|---|---|
| 1 | 6 × (1²) = 6 | 1³ = 1 | 6 : 1 |
| 2 | 6 × (2²) = 24 | 2³ = 8 | 3 : 1 |
| 4 | 6 × (4²) = 96 | 4³ = 64 | 1.5 : 1 |
Observation: As side length increases, SA:V ratio decreases.
📊 4. Calculating SA:V for a Sphere
- Formula: SA = 4 × π × r², V = (4/3) × π × r³
| Radius (cm) | Surface Area (cm²) | Volume (cm³) | SA:V Ratio |
|---|---|---|---|
| 1 | 4 × 3.14 × (1²) = 12.56 | (4/3) × 3.14 × 1³ = 4.19 | 3 : 1 |
| 2 | 4 × 3.14 × (2²) = 50.24 | (4/3) × 3.14 × 8 = 33.51 | 1.5 : 1 |
| 4 | 4 × 3.14 × (4²) = 201.06 | (4/3) × 3.14 × 64 = 268.08 | 0.75 : 1 |
As objects get bigger, their SA:V ratio decreases.
Small cells are more efficient at exchanging substances.
This explains why most cells are microscopic – large cells struggle to get enough nutrients and remove wastes quickly.
Investigation: Effect of SA:V Ratio on Diffusion in Agar Blocks
🌱 Aim
- To investigate: How changing the surface area to volume (SA:V) ratio affects the rate of diffusion using agar blocks.
🧬 Background
- Diffusion: Movement of particles from high concentration to low concentration.
- SA:V effect: Higher SA:V → faster net exchange/penetration of substances; lower SA:V → slower penetration.
- Prediction: Smaller agar blocks (larger SA:V) will lose colour faster when placed in acid.
🧪 Materials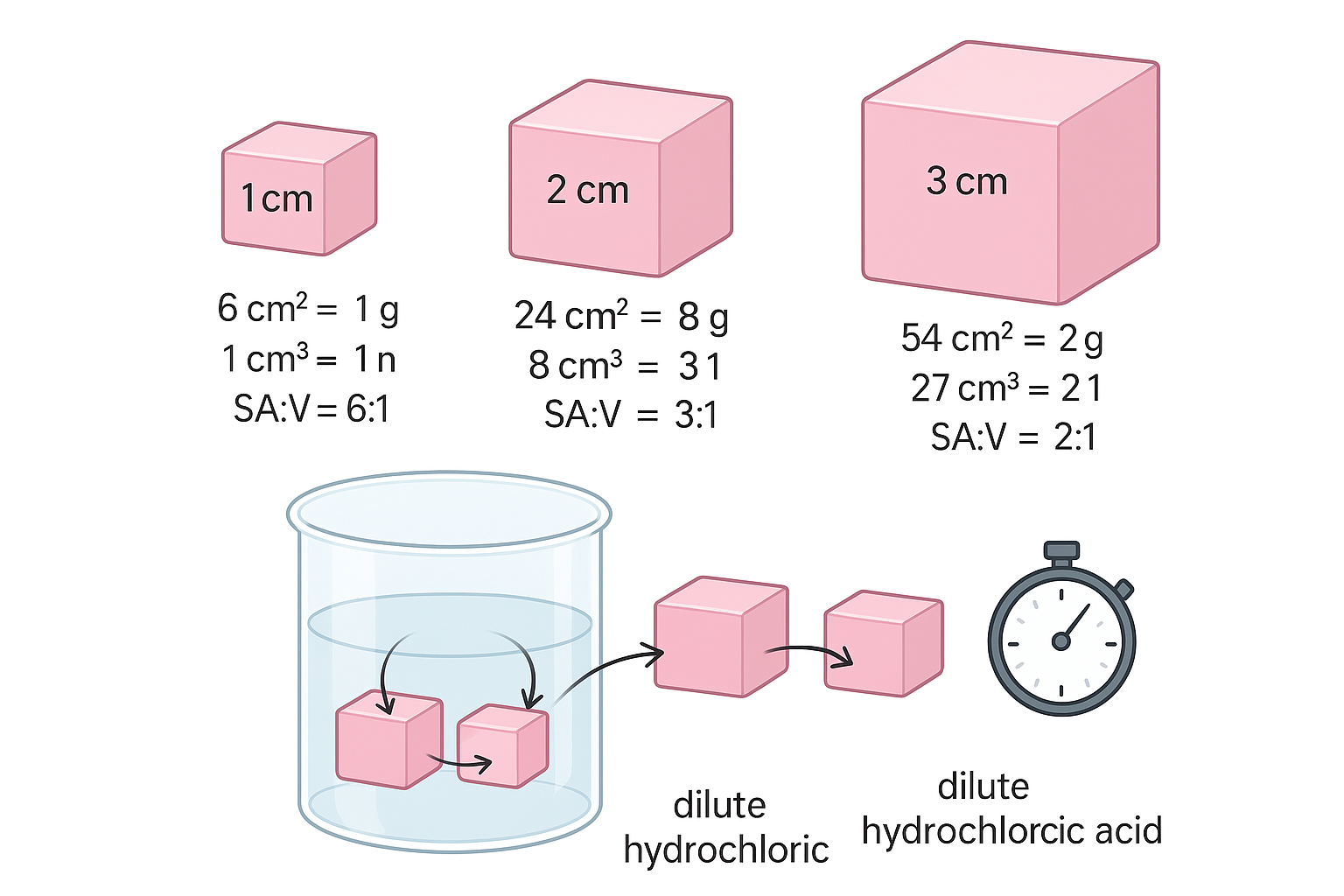
- Agar blocks containing a pH indicator (e.g., phenolphthalein)
- Beaker of dilute acid (e.g., 0.1 M HCl)
- Ruler
- Scalpel or knife
- Stopwatch
- Marker / labels
🔬 Method
- Prepare agar blocks of different cube sizes (e.g., side lengths 1 cm, 2 cm, 3 cm).
- Measure and record the dimensions of each block precisely.
- Calculate surface area (SA), volume (V), and SA:V ratio for each block.
- Place all blocks in the dilute HCl at the same time and start the stopwatch.
- Record the time taken for each block to become fully colourless (indicator neutralised).
- Repeat the experiment (at least 2–3 trials) and calculate mean times.
- Compare times against SA:V ratios and draw conclusions.
📊 Example Calculations (Cube-shaped blocks)
| Side Length (cm) | SA (cm²) | Volume (cm³) | SA:V Ratio | Time for Colour to Disappear (min) |
|---|---|---|---|---|
| 1 | 6 × (1²) = 6 | 1³ = 1 | 6 : 1 | 2.0 |
| 2 | 6 × (2²) = 24 | 2³ = 8 | 3 : 1 | 5.0 |
| 3 | 6 × (3²) = 54 | 3³ = 27 | 2 : 1 | 9.0 |
Observation: As SA:V decreases (blocks get larger), the time for the acid to diffuse and neutralise the indicator increases.
🧠 Conclusion
- Smaller agar blocks (higher SA:V) allow faster penetration of acid and quicker colour change.
- Larger blocks have proportionally less surface per unit volume, so diffusion takes longer to reach the centre.
- This supports why biological cells are small — high SA:V enables efficient exchange of substances required for metabolism.
High SA:V → faster diffusion rate.
Large size → low SA:V → slower exchange.
Calculate SA:V to compare efficiency between different shapes/sizes.
Repeat trials and use mean times to improve reliability.
Investigation: Effect of Water Potential on Plant Tissue
Aim
- To investigate how plant tissue mass changes when immersed in solutions of different water potentials.
- To use results to estimate the tissue’s water potential.
🧬 Background Concepts
- Water potential (Ψ): Measure of the tendency of water to move from one region to another (kPa).
- Water moves from higher Ψ (less negative) → lower Ψ (more negative).
- Osmosis: Passive movement of water across a partially permeable membrane.
- Hypotonic solution: Water enters cells → cells become turgid.
- Hypertonic solution: Water leaves cells → cells become plasmolysed.
🧪 Materials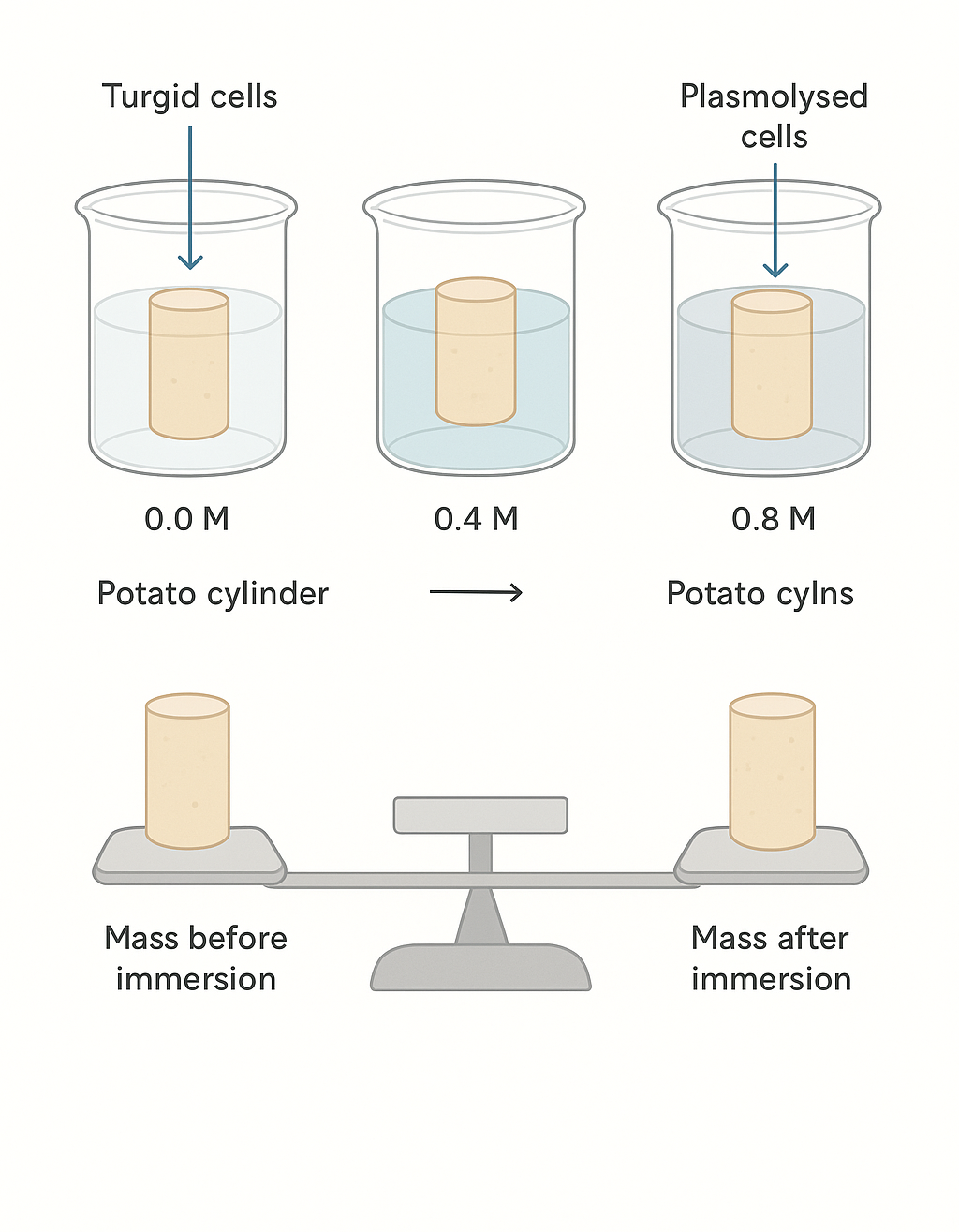
- Fresh potato or beetroot cylinders (equal size)
- Cork borer or scalpel
- Distilled water
- Sucrose solutions of known concentrations (0.0 M, 0.2 M, 0.4 M, 0.6 M, 0.8 M)
- Beakers/test tubes
- Balance (0.01 g accuracy)
- Tissue paper
- Ruler
🔬 Method
- Cut plant tissue cylinders to equal length and diameter.
- Measure and record the initial mass of each cylinder.
- Place each in a separate beaker/test tube with a different sucrose concentration.
- Leave for a fixed time (30–60 minutes).
- Remove, blot gently with tissue paper.
- Measure final mass.
- Calculate % change in mass using:
% change = ((final mass − initial mass) ÷ initial mass) × 100
- Plot % change in mass vs sucrose concentration.
📊 Example Results Table
| Sucrose Conc. (M) | Initial Mass (g) | Final Mass (g) | % Change in Mass |
|---|---|---|---|
| 0.0 | 1.20 | 1.34 | +11.7% |
| 0.2 | 1.25 | 1.30 | +4.0% |
| 0.4 | 1.28 | 1.28 | 0.0% |
| 0.6 | 1.24 | 1.19 | -4.0% |
| 0.8 | 1.27 | 1.18 | -7.1% |
📈 Data Interpretation
- Low sucrose conc. (high Ψ) → tissue gains mass (water enters).
- High sucrose conc. (low Ψ) → tissue loses mass (water leaves).
- The concentration where % change = 0 → tissue Ψ is equal to solution Ψ.
- Use standard Ψ values for sucrose to estimate tissue water potential.
🧠 Conclusion
- Tissue water potential can be estimated from the point of no net mass change.
- Demonstrates osmosis and how water potential differences drive water movement.
– Water potential gradient drives osmosis.
– Mass gain/loss depends on whether external Ψ is higher or lower than tissue Ψ.
– Graphical method provides more accurate Ψ estimates.
Movement of Water Between Cells and Solutions
🌱 Water Potential (Ψ)
- Definition: A measure of the tendency of water molecules to move from one place to another.
- Rule: Water moves from a higher water potential (less negative) → to a lower water potential (more negative).
- Pure water: Highest water potential (Ψ = 0).
🔄 Water Movement Scenarios
| External Solution | Water Potential Difference | Movement of Water | Effect on Plant Cells | Effect on Animal Cells |
|---|---|---|---|---|
| Hypotonic (higher Ψ outside cell) | Outside > Inside | Water enters cell | Becomes turgid (cell wall prevents bursting) | Swells and may burst (lysis) – no cell wall |
| Isotonic (same Ψ inside & outside) | Equal | No net water movement | Cell remains flaccid (no net change) | Cell remains normal shape |
| Hypertonic (lower Ψ outside cell) | Outside < Inside | Water leaves cell | Becomes plasmolysed (cytoplasm shrinks away from cell wall) | Shrinks and becomes crenated |
🧬 Effects on Cells
- Plant cells: Protected by a rigid cell wall – become turgid in hypotonic solutions rather than bursting; plasmolyse in hypertonic solutions as the plasma membrane pulls away from the wall.
- Animal cells: Lack a cell wall – sensitive to water potential changes; may swell and lyse in hypotonic solutions or shrink (crenate) in hypertonic solutions.
Water potential (Ψ) determines the direction of water movement.
Plant cells resist bursting due to the cell wall but can plasmolyse in hypertonic media.
Animal cells readily change shape and may burst or shrink depending on the surrounding solution.
