CIE AS/A Level Biology -8.2 Transport of oxygen and carbon dioxide- Study Notes- New Syllabus
CIE AS/A Level Biology -8.2 Transport of oxygen and carbon dioxide- Study Notes- New Syllabus
Ace A level Biology Exam with CIE AS/A Level Biology -8.2 Transport of oxygen and carbon dioxide- Study Notes- New Syllabus
Key Concepts:
- describe the role of red blood cells in transporting oxygen and carbon dioxide with reference to the roles of:
• haemoglobin
• carbonic anhydrase
• the formation of haemoglobinic acid
• the formation of carbaminohaemoglobin - describe the chloride shift and explain the importance of the chloride shift
- describe the role of plasma in the transport of carbon dioxide
- describe and explain the oxygen dissociation curve of adult haemoglobin
- explain the importance of the oxygen dissociation curve at partial pressures of oxygen in the lungs and in respiring tissues
- describe the Bohr shift and explain the importance of the Bohr shift
Transport of Oxygen and Carbon Dioxide by Red Blood Cells (RBCs)
🌱 Overview
- Red blood cells (RBCs) are specialised for gas transport in mammals.
- Key roles involve haemoglobin, carbonic anhydrase, and formation of haemoglobinic acid and carbaminohaemoglobin.
🔬 1. Role of Haemoglobin (Hb)
- Definition: A protein in RBCs containing iron that binds gases.
- Oxygen transport:
- Each Hb molecule can bind 4 oxygen molecules → forms oxyhaemoglobin (HbO₂).
- Facilitates efficient oxygen delivery from lungs to tissues.
- Carbon dioxide transport:
- Hb binds some CO₂ directly to form carbaminohaemoglobin (HbCO₂).
🔬 2. Role of Carbonic Anhydrase
- Enzyme present in RBCs.
- Catalyses reaction: CO₂ + H₂O ⇌ H₂CO₃
- Converts carbon dioxide + water → carbonic acid (H₂CO₃) quickly.
- Carbonic acid dissociates into bicarbonate (HCO₃⁻) and hydrogen ions (H⁺): H₂CO₃ ⇌ H⁺ + HCO₃⁻
- Function: Enables rapid transport of CO₂ in blood plasma.
🔬 3. Formation of Haemoglobinic Acid (HHb)
- Definition: Haemoglobin bound to hydrogen ions.
- Reaction: Hb + H⁺ → HHb
- Function: Buffers blood pH and prevents acidification from CO₂ accumulation in tissues.
🔬 4. Formation of Carbaminohaemoglobin (HbCO₂)
- Definition: Haemoglobin bound to carbon dioxide (CO₂) at amino groups.
- Reaction: Hb + CO₂ → HbCO₂
- Function: Transports ~20–25% of CO₂ from tissues to lungs, allowing efficient removal of CO₂ from metabolically active tissues.
📊 Summary Table: RBC Roles in Gas Transport
| Component | Reaction / Mechanism | Function |
|---|---|---|
| Haemoglobin (Hb) | Hb + O₂ → HbO₂ | Transports oxygen to tissues |
| Carbonic anhydrase | CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻ | Rapid CO₂ transport as bicarbonate |
| Haemoglobinic acid (HHb) | Hb + H⁺ → HHb | Buffers blood, carries H⁺ ions |
| Carbaminohaemoglobin (HbCO₂) | Hb + CO₂ → HbCO₂ | Transports CO₂ (~20–25%) from tissues to lungs |
– RBCs transport oxygen via oxyhaemoglobin and CO₂ via bicarbonate and carbaminohaemoglobin.
– Carbonic anhydrase speeds up CO₂ conversion to bicarbonate.
– Haemoglobinic acid formation helps buffer blood pH, ensuring homeostasis.
– Overall, RBCs efficiently maintain gas exchange and acid-base balance in mammals.
Chloride Shift – Mechanism and Importance
🌱 Overview
- The chloride shift (also called the Hamburger phenomenon) occurs in red blood cells (RBCs) during carbon dioxide transport.
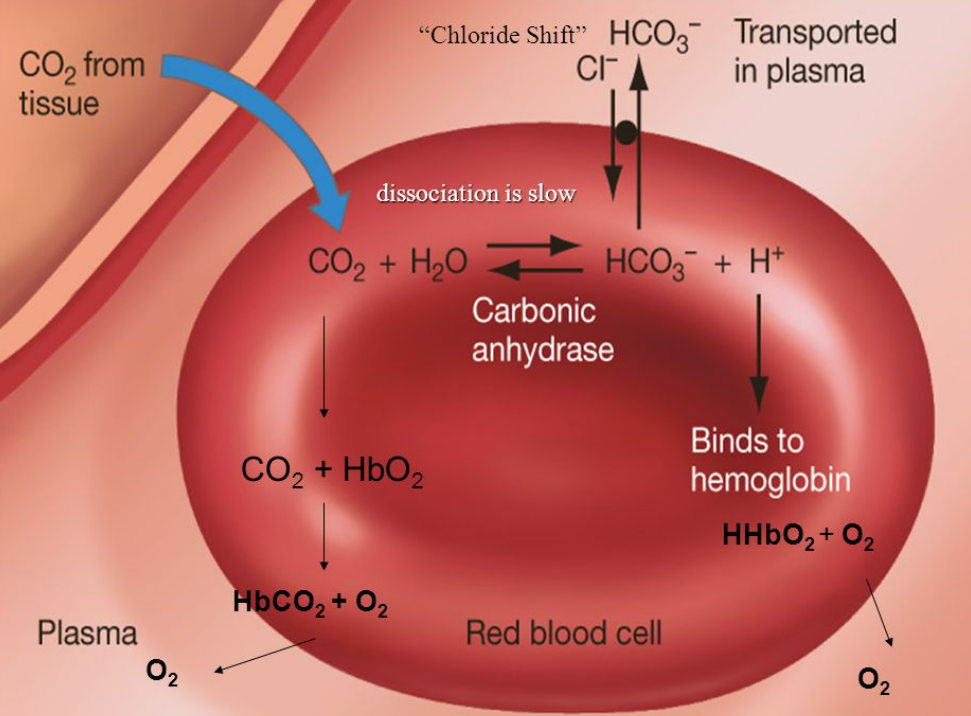 It helps maintain electrical neutrality when bicarbonate ions (HCO₃⁻) move out of RBCs into plasma.
It helps maintain electrical neutrality when bicarbonate ions (HCO₃⁻) move out of RBCs into plasma.
🔬 Mechanism of Chloride Shift
- CO₂ enters RBCs from tissues.
- CO₂ diffuses into RBCs and reacts with water, catalysed by carbonic anhydrase:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻ - Bicarbonate ions (HCO₃⁻) diffuse out into plasma.
- To maintain electrical neutrality, chloride ions (Cl⁻) move into RBCs from plasma.
- Hydrogen ions (H⁺) are buffered by binding to haemoglobin, forming haemoglobinic acid (HHb), preventing blood acidification.
- At the lungs, the process is reversed: HCO₃⁻ moves back into RBCs, Cl⁻ exits, allowing CO₂ to be released into alveoli.
📊 Importance of Chloride Shift
| Importance | Explanation |
|---|---|
| Maintains electrical neutrality | Prevents charge imbalance as HCO₃⁻ leaves RBCs and Cl⁻ enters |
| Facilitates CO₂ transport | Allows continuous conversion of CO₂ to HCO₃⁻ for efficient blood transport |
| Supports acid-base balance | H⁺ ions are buffered by haemoglobin, preventing blood pH changes |
| Ensures efficient gas exchange | Enables CO₂ removal at lungs and oxygen loading at tissues |
– Chloride shift occurs mainly in tissue capillaries and is reversed at the lungs.
– Essential for efficient CO₂ transport in plasma without disrupting RBC function or blood pH.
– Maintains homeostasis and ensures rapid gas exchange during circulation.
Role of Plasma in Transport of Carbon Dioxide
🌱 Overview
- Plasma is the liquid component of blood (~55% of blood volume).
- It transports CO₂ from tissues to the lungs, along with other substances like nutrients and wastes.
🔬 Mechanisms of CO₂ Transport in Plasma
- Dissolved CO₂ (~5–10%)
A small proportion of CO₂ dissolves directly in plasma.
Diffuses freely from tissues to plasma and then to the lungs. - Bicarbonate ions (HCO₃⁻, ~85–90%)
CO₂ enters RBCs and is converted to bicarbonate ions by carbonic anhydrase:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻
HCO₃⁻ diffuses into plasma for transport to lungs.
This is the main way CO₂ is carried in plasma. - Carbamino compounds (~5–10%)
CO₂ binds to plasma proteins (mainly globulins) to form carbamino compounds.
Plays a minor role in CO₂ transport.
📊 Summary Table: CO₂ Transport via Plasma
| Method | Approx. % of CO₂ Transported | Description |
|---|---|---|
| Dissolved CO₂ | 5–10% | CO₂ directly dissolved in plasma |
| Bicarbonate ions (HCO₃⁻) | 85–90% | Main transport form; formed in RBCs, diffuses into plasma |
| Carbamino compounds | 5–10% | CO₂ binds to plasma proteins (globulins) |
– Plasma acts as a transport medium for CO₂, both dissolved and as bicarbonate.
– Conversion of CO₂ to HCO₃⁻ in RBCs allows greater CO₂ transport than simple dissolution.
– Maintains blood pH and ensures efficient CO₂ removal at the lungs.
Oxygen Dissociation Curve of Adult Haemoglobin
🌱 Overview
- The oxygen dissociation curve shows the relationship between oxygen partial pressure (pO₂) and the percentage saturation of haemoglobin (Hb) with oxygen.
- It illustrates how efficiently haemoglobin binds and releases oxygen under different conditions.
🔬 Shape of the Curve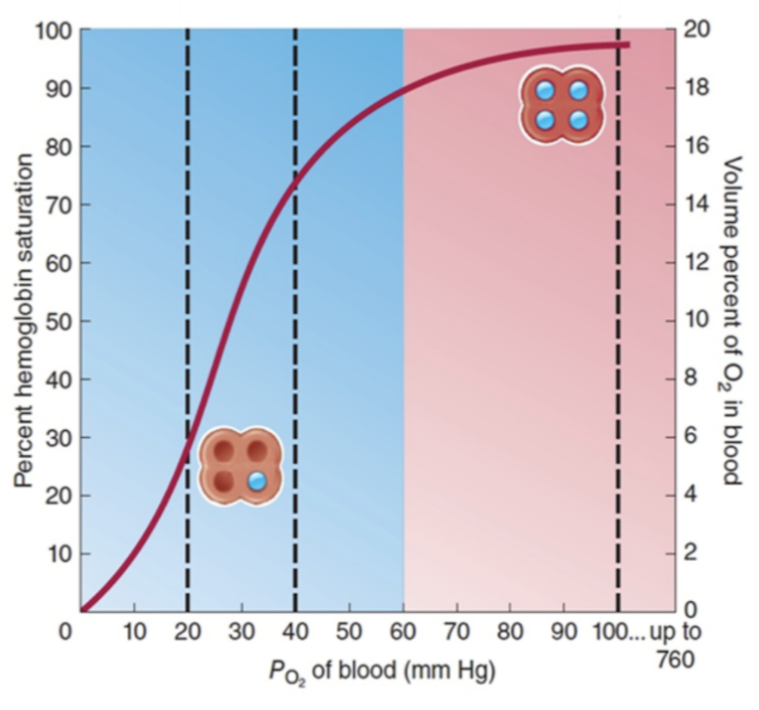
- The curve is sigmoid (S-shaped).
- Reason for S-shape: Cooperative binding: when one O₂ binds to Hb, the affinity of Hb for subsequent O₂ molecules increases. After three O₂ molecules bind, the fourth binds more easily.
- At low pO₂ → Hb has low saturation (steep initial slope).
- At high pO₂ → Hb approaches full saturation (plateau phase).
🔬 Interpretation of Key Points
- Steep Portion (Low pO₂, tissues): Small drop in pO₂ → large release of O₂ from Hb. Ensures oxygen delivery to respiring tissues.
- Plateau Portion (High pO₂, lungs): Hb nearly saturated at high pO₂ → efficient oxygen loading in lungs.
- P50 Value: pO₂ at which Hb is 50% saturated. Indicates Hb’s affinity for oxygen: lower P50 → higher affinity; higher P50 → lower affinity.
🔬 Factors Affecting the Curve
- Right Shift (Bohr Effect) – reduces Hb affinity → more O₂ released to tissues
- Increased CO₂
- Increased H⁺ (lower pH)
- Increased temperature
- Left Shift – increases Hb affinity → O₂ held more tightly
- Decreased CO₂
- Increased pH
- Lower temperature
📊 Summary Table: Oxygen Dissociation Curve Features
| Feature | Description / Function |
|---|---|
| Sigmoid shape | Shows cooperative binding of O₂ to Hb |
| Steep portion | Facilitates O₂ release to tissues |
| Plateau portion | Ensures O₂ uptake in lungs |
| P50 | O₂ partial pressure at 50% Hb saturation; measures affinity |
| Right shift | More O₂ released; occurs at high CO₂, low pH, high temp |
| Left shift | Hb holds O₂ more tightly; occurs at low CO₂, high pH, low temp |
– The S-shaped curve reflects cooperative O₂ binding of haemoglobin.
– Hb loads O₂ efficiently at lungs (high pO₂) and releases O₂ where needed in tissues (low pO₂).
– Shifts in the curve adapt oxygen delivery to metabolic needs.
Importance of the Oxygen Dissociation Curve at Different pO₂
🌱 Overview
- The oxygen dissociation curve shows how haemoglobin (Hb) binds and releases oxygen at different partial pressures of oxygen (pO₂).
- Its shape is critical for oxygen uptake in the lungs and oxygen release in respiring tissues.
🔬 1. At High pO₂ (Lungs)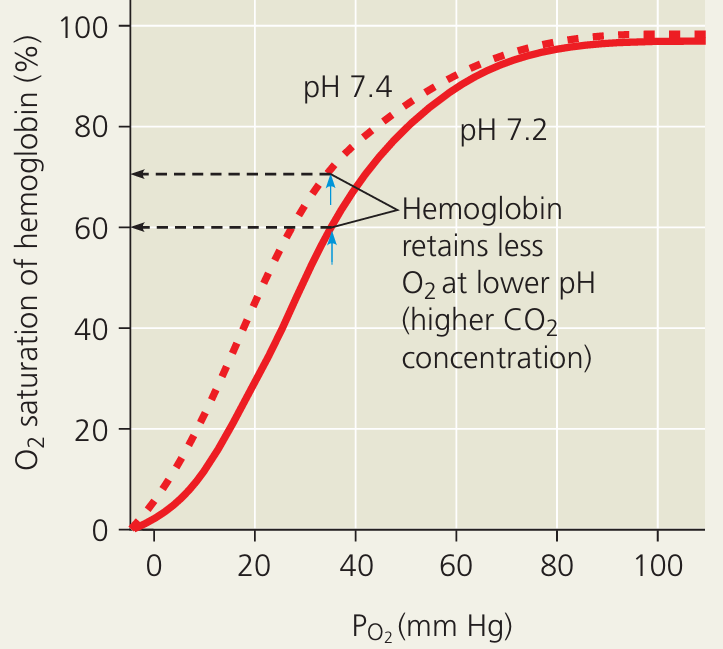
- pO₂ in alveoli: ~100 mmHg
- Curve is in the plateau phase → Hb is nearly fully saturated (~97–100%)
- Importance:
- Ensures maximum oxygen loading in the lungs, even if pO₂ fluctuates slightly.
- Provides a safety margin for efficient oxygen uptake.
🔬 2. At Low pO₂ (Respiring Tissues)
- pO₂ in tissues: ~20–40 mmHg (varies with activity)
- Curve is in the steep portion → small drop in pO₂ → large release of O₂ from Hb
- Importance:
- Provides rapid oxygen delivery to tissues with high metabolic activity.
- Ensures cells get enough oxygen for aerobic respiration.
📊 Summary Table: Function of Curve at Different pO₂
| Location | pO₂ (mmHg) | Curve Region | Hb Saturation | Functional Importance |
|---|---|---|---|---|
| Lungs | ~100 | Plateau | ~97–100% | Efficient oxygen loading; safety margin |
| Tissues (resting) | ~40 | Steep slope | ~75% | Rapid O₂ release for metabolism |
| Tissues (active) | ~20 | Steep slope | ~30–40% | Meets high oxygen demand |
– Plateau region at lungs: maximises oxygen uptake even if pO₂ drops slightly.
– Steep region at tissues: facilitates oxygen unloading where it is most needed.
– The curve ensures efficient oxygen delivery to support metabolism at rest and during activity.
Bohr Shift – Mechanism and Importance
🌱 Overview
- The Bohr shift (Bohr effect) describes how haemoglobin’s affinity for oxygen decreases in the presence of high CO₂ concentration or low pH.
- This rightward shift in the oxygen dissociation curve facilitates oxygen delivery to actively respiring tissues.
🔬 Mechanism of the Bohr Shift
- CO₂ enters tissues from metabolically active cells.
- CO₂ combines with water to form carbonic acid (H₂CO₃) via carbonic anhydrase:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻ - Hydrogen ions (H⁺) increase in tissues.
- H⁺ ions bind to haemoglobin → formation of haemoglobinic acid (HHb), reducing Hb’s affinity for oxygen.
- Oxygen is released more readily; Hb releases O₂ at lower pO₂ → more oxygen reaches tissues with high metabolic activity.
🔬 Importance of the Bohr Shift
| Feature | Explanation |
|---|---|
| Enhances oxygen delivery | Tissues producing more CO₂ (active muscles) receive more oxygen when needed. |
| Matches oxygen supply to demand | High metabolic activity → more CO₂ → more O₂ released → efficient energy production. |
| Prevents unnecessary oxygen retention | Oxygen is released only where it is needed, maintaining efficiency. |
| Facilitates CO₂ transport | Formation of HHb buffers H⁺, aiding CO₂ transport as bicarbonate. |
📊 Summary Table: Bohr Shift Effects
| Condition | Curve Shift | Hb Affinity for O₂ | Physiological Outcome |
|---|---|---|---|
| High CO₂ / low pH (tissues) | Right shift | Decreased | More O₂ released to active tissues |
| Low CO₂ / high pH (lungs) | Left shift | Increased | Hb binds O₂ efficiently in lungs |
– The Bohr shift ensures oxygen delivery matches tissue metabolic demand.
– Right shift occurs in tissues with high CO₂ and low pH; left shift occurs in lungs where CO₂ is low.
– Critical for efficient respiration and energy production in mammals.
