CIE AS/A Level Chemistry 11.1 Physical properties of the Group 17 elements Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 11.1 Physical properties of the Group 17 elements Study Notes – New Syllabus
CIE AS/A Level Chemistry 11.1 Physical properties of the Group 17 elements Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe colours and volatility trends of Cl₂, Br₂ and I₂
explain trends in bond strength
interpret volatility using intermolecular forces
Colours and Volatility of Chlorine, Bromine and Iodine
Chlorine, bromine and iodine are Group 17 elements (the halogens). They exist as diatomic molecules and show clear trends in colour and volatility down the group due to increasing molecular size and stronger intermolecular forces.
Colours of the Halogens
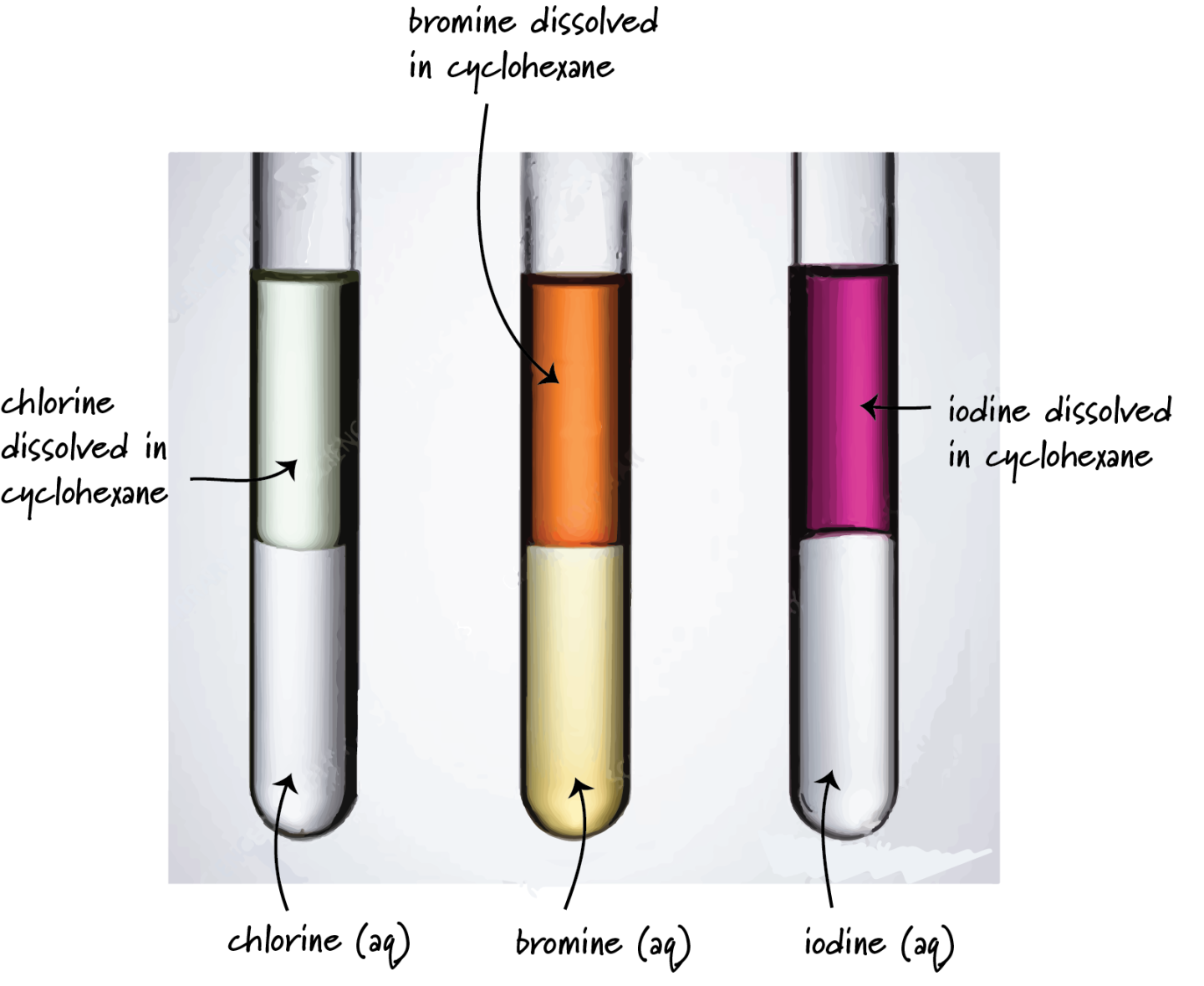
- Chlorine, \( \mathrm{Cl_2} \): pale green gas
- Bromine, \( \mathrm{Br_2} \): red-brown liquid
- Iodine, \( \mathrm{I_2} \): dark grey solid (purple vapour when heated)
Trend: The colour of the halogens becomes darker down the group.
Explanation: As atomic number increases, electrons occupy higher energy levels and the halogens absorb lower-energy (longer-wavelength) visible light. This causes the observed colour to deepen from chlorine to iodine.
Volatility of the Halogens
Volatility is a measure of how easily a substance evaporates.

Trend: \( \mathrm{Cl_2 > Br_2 > I_2} \)
Explanation:
- Halogen molecules are held together by London dispersion forces
- Down the group, molecular size and number of electrons increase
- Electron clouds become more easily polarised
- London dispersion forces become stronger
- More energy is required to separate the molecules
As a result, boiling points increase and volatility decreases down the group, changing from gas (chlorine) to liquid (bromine) to solid (iodine) at room temperature.
Example
State the colour and physical state of chlorine and iodine at room temperature.
▶️ Answer / Explanation
Chlorine is a pale green gas.
Iodine is a dark grey solid (which forms a purple vapour when heated).
Example
Explain why bromine is less volatile than chlorine.
▶️ Answer / Explanation
Bromine molecules are larger and contain more electrons than chlorine molecules.
This increases the strength of London dispersion forces between bromine molecules.
More energy is required to overcome these forces, so bromine has a higher boiling point and is less volatile than chlorine.
Example
Describe and explain the trends in colour and volatility from chlorine to iodine.
▶️ Answer / Explanation
The colour of the halogens darkens from pale green chlorine to red-brown bromine to dark grey iodine.
This is due to changes in electronic transitions as atomic size and electron number increase.
Volatility decreases down the group because increasing molecular size strengthens London dispersion forces.
As a result, boiling points increase and the physical state changes from gas to liquid to solid.
Bond Strength of Halogen Molecules
The halogens exist as simple diatomic molecules, \( \mathrm{X_2} \), where a single covalent bond joins two halogen atoms. The strength of this bond shows a clear trend down Group 17.
Trend in Bond Strength
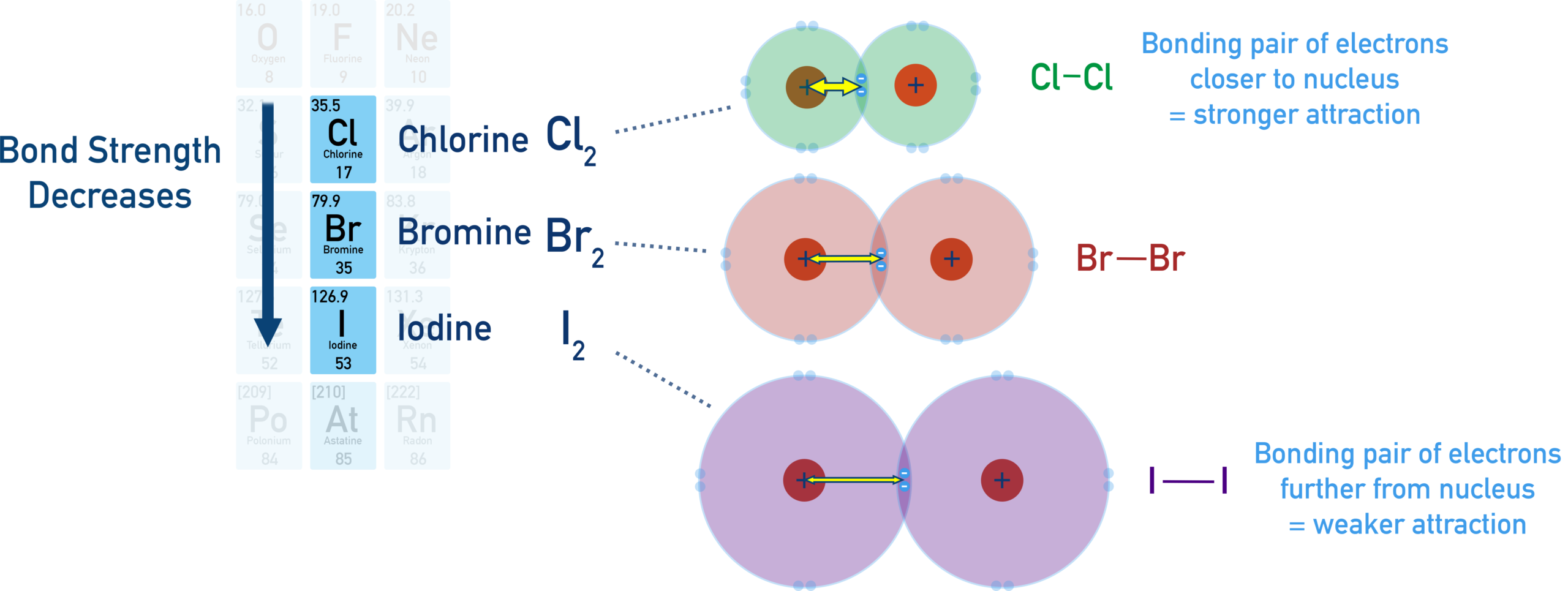
Bond strength decreases down the group:
\( \mathrm{Cl_2 > Br_2 > I_2} \)
Explanation of the Trend
- Down Group 17, atomic radius increases
- Bond length between the two halogen atoms increases
- The bonding pair of electrons is further from the positively charged nuclei
- There is greater shielding by inner electron shells
- The electrostatic attraction between the nuclei and the bonding electrons decreases
As a result, the covalent bond becomes weaker down the group, so less energy is required to break the \( \mathrm{X–X} \) bond.
This is why the bond enthalpy of the halogen molecules decreases from chlorine to iodine.
Example
State the order of bond strength for the molecules \( \mathrm{Cl_2} \), \( \mathrm{Br_2} \) and \( \mathrm{I_2} \).
▶️ Answer / Explanation
\( \mathrm{Cl_2 > Br_2 > I_2} \)
Example
Explain why the bond in \( \mathrm{Br_2} \) is weaker than the bond in \( \mathrm{Cl_2} \).
▶️ Answer / Explanation
Bromine atoms are larger than chlorine atoms, so the bond length in \( \mathrm{Br_2} \) is longer.
The bonding electrons are further from the nuclei and experience greater shielding.
This reduces the electrostatic attraction between the nuclei and the bonding pair, making the bond weaker.
Example
Describe and explain the trend in the bond enthalpy of halogen molecules down Group 17.
▶️ Answer / Explanation
The bond enthalpy of halogen molecules decreases down Group 17.
As atomic radius increases, bond length increases and the bonding pair of electrons is further from the nuclei.
Increased shielding from inner shells reduces nuclear attraction to the bonding electrons.
This weakens the covalent bond, so less energy is required to break it from chlorine to iodine.
Volatility of Elements in Terms of Instantaneous Dipole–Induced Dipole Forces
Non-polar elements such as the halogens exist as simple molecules and are held together by instantaneous dipole–induced dipole forces, also known as London dispersion forces. The volatility of these elements can be directly interpreted using the strength of these forces.
Instantaneous Dipole–Induced Dipole Forces
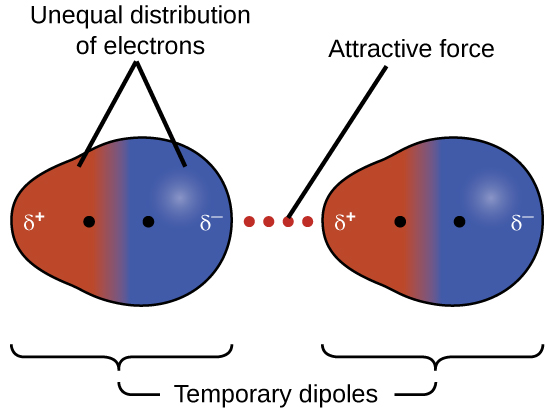
- Electrons within a molecule are constantly moving
- At any moment, the electron density may be uneven
- This creates an instantaneous (temporary) dipole
- The instantaneous dipole induces a dipole in a neighbouring molecule
- An attractive force forms between the two molecules
Interpretation of Volatility

Trend in volatility (halogens): \( \mathrm{Cl_2 > Br_2 > I_2} \)
Explanation of the Trend
- Down the group, atoms and molecules become larger
- The number of electrons in each molecule increases
- Electron clouds become more easily polarised
- Instantaneous dipoles formed are larger
- Induced dipoles in neighbouring molecules are stronger
- Overall instantaneous dipole–induced dipole forces increase
As the strength of instantaneous dipole–induced dipole forces increases, more energy is required to separate the molecules. This leads to higher boiling points and therefore lower volatility down the group.
Chlorine has the weakest instantaneous dipole–induced dipole forces and is therefore the most volatile, whereas iodine has the strongest forces and is the least volatile.
Example
Name the intermolecular force responsible for the volatility of the halogens.
▶️ Answer / Explanation
Instantaneous dipole–induced dipole forces (London dispersion forces).
Example
Explain why iodine is less volatile than bromine.
▶️ Answer / Explanation
Iodine molecules have more electrons and a larger electron cloud than bromine molecules.
This makes the electron cloud more easily polarised, leading to stronger instantaneous dipole–induced dipole forces.
More energy is required to overcome these forces, so iodine is less volatile.
Example
Using ideas about intermolecular forces, explain why chlorine is a gas while iodine is a solid at room temperature.
▶️ Answer / Explanation
Chlorine molecules are smaller with fewer electrons, so they form weaker instantaneous dipole–induced dipole forces.
Only a small amount of energy is needed to overcome these forces, so chlorine is a gas.
Iodine molecules are much larger and have more electrons, making their electron clouds highly polarised.
This results in strong instantaneous dipole–induced dipole forces that require much more energy to overcome.
Therefore iodine is a solid at room temperature.
