CIE AS/A Level Chemistry 11.2 The chemical properties of the halogen elements and the hydrogen halides Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 11.2 The chemical properties of the halogen elements and the hydrogen halides Study Notes – New Syllabus
CIE AS/A Level Chemistry 11.2 The chemical properties of the halogen elements and the hydrogen halides Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe oxidising power trends
describe reactions with hydrogen
describe thermal stability of hydrogen halides
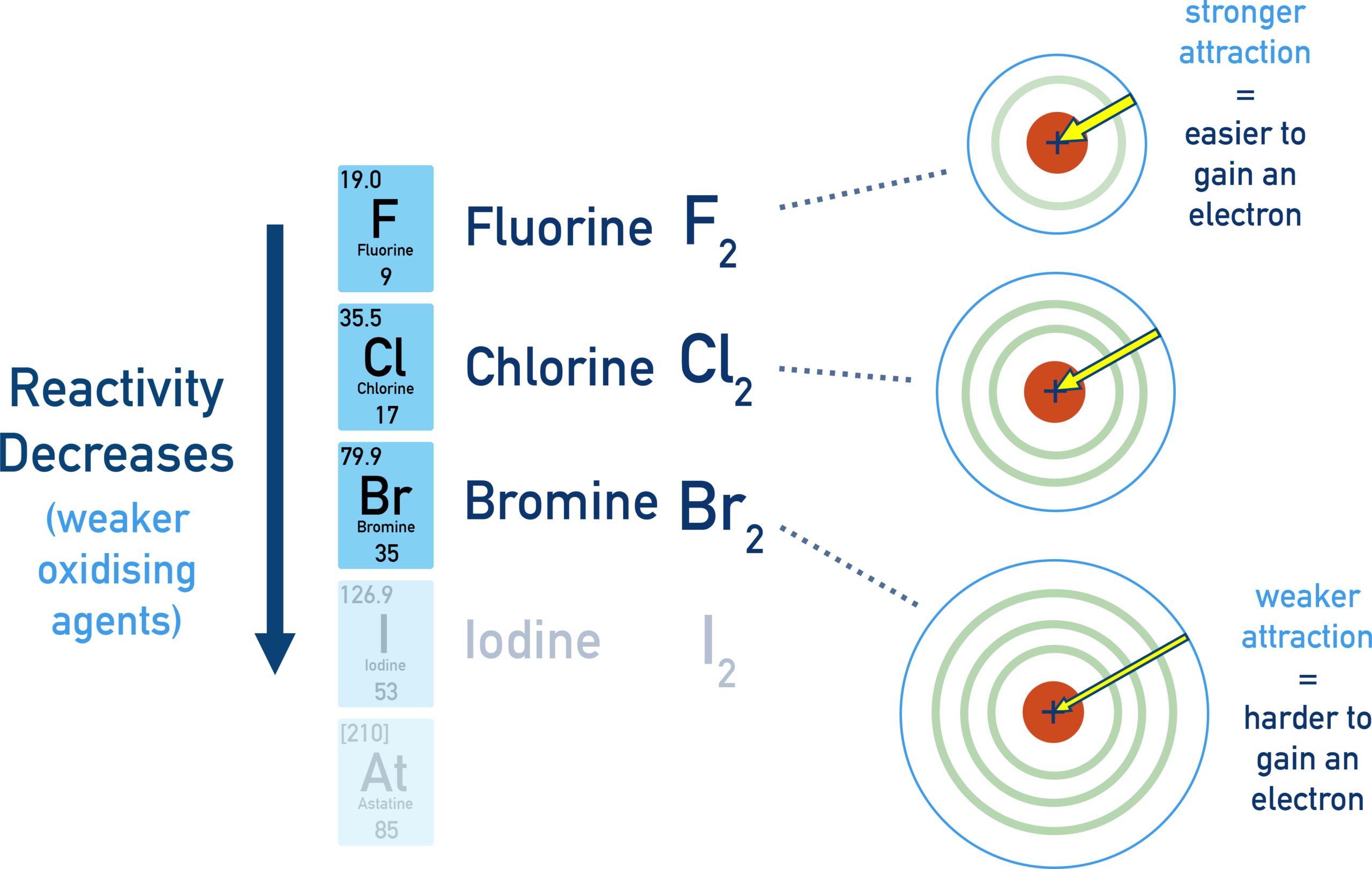
Relative Reactivity of the Halogens as Oxidising Agents
The halogens act as oxidising agents because they gain electrons to form halide ions. The ability of a halogen to oxidise another substance depends on how easily it gains electrons.
Oxidising Agent Behaviour
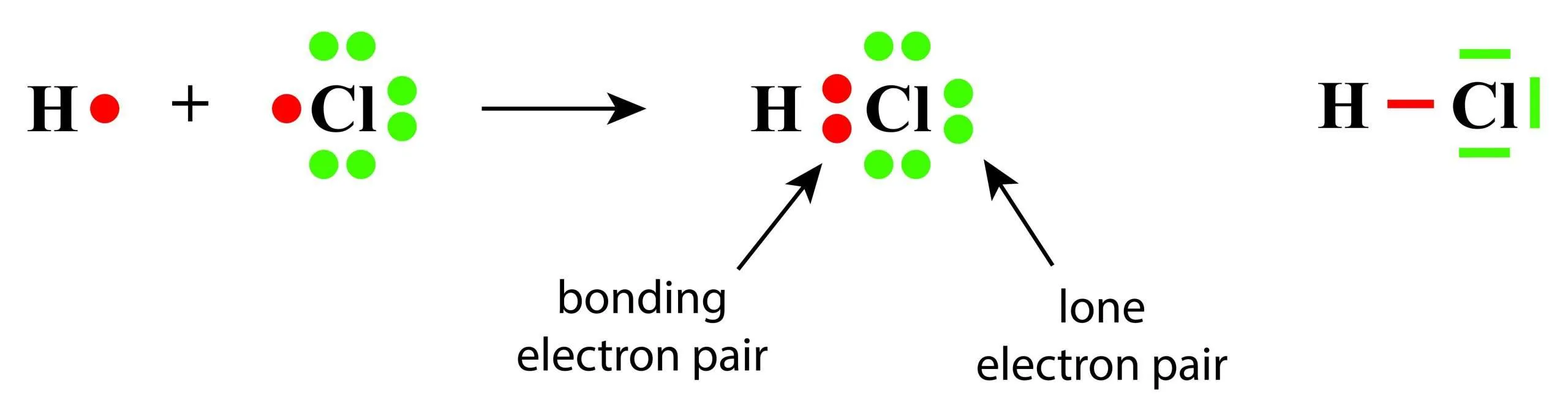
A halogen molecule is reduced by gaining electrons:
\( \mathrm{X_2 + 2e^- \rightarrow 2X^-} \)
Relative Reactivity (Oxidising Strength)
Trend: \( \mathrm{Cl_2 > Br_2 > I_2} \)
Chlorine is the strongest oxidising agent, followed by bromine, while iodine is the weakest.
Explanation of the Trend
- Down Group 17, atomic radius increases
- The outer shell is further from the nucleus
- There is increased shielding by inner electron shells
- The attraction between the nucleus and incoming electrons decreases
- Halogens gain electrons less readily down the group
As a result, the ability of the halogens to act as oxidising agents decreases down the group.
Displacement Reactions (Evidence of Oxidising Strength)
A more reactive halogen can oxidise a halide ion of a less reactive halogen:
\( \mathrm{Cl_2 + 2Br^- \rightarrow 2Cl^- + Br_2} \)
Chlorine displaces bromine and iodine from their aqueous halide solutions. Bromine can displace iodine but not chlorine. Iodine cannot displace chlorine or bromine.
Example
State which halogen is the strongest oxidising agent.
▶️ Answer / Explanation
Chlorine is the strongest oxidising agent.
Example
Explain why bromine is a weaker oxidising agent than chlorine.
▶️ Answer / Explanation
Bromine atoms are larger than chlorine atoms and have greater shielding.
This reduces the attraction between the nucleus and incoming electrons.
Therefore bromine gains electrons less readily and is a weaker oxidising agent.
Example
Using ideas about electron transfer, explain why iodine cannot oxidise bromide ions but chlorine can.
▶️ Answer / Explanation
Chlorine atoms are smaller with less shielding, so they have a stronger attraction for electrons.
Chlorine molecules gain electrons more readily and can oxidise bromide ions to bromine.
Iodine atoms are larger and more shielded, so they gain electrons less readily.
As a result, iodine cannot oxidise bromide ions.
Reactions of the Halogens with Hydrogen
The halogens react with hydrogen to form hydrogen halides, \( \mathrm{HX} \). The ease and conditions of these reactions show a clear trend in reactivity down Group 17.
General Reaction
\( \mathrm{H_2 + X_2 \rightarrow 2HX} \)
Reactions of Individual Halogens
- Chlorine reacts readily with hydrogen, explosively in sunlight, to form hydrogen chloride
- Bromine reacts more slowly and requires heating or a catalyst (e.g. platinum)
- Iodine reacts very slowly and the reaction is reversible, forming an equilibrium mixture
Example equations:
\( \mathrm{H_2 + Cl_2 \rightarrow 2HCl} \)
\( \mathrm{H_2 + Br_2 \rightarrow 2HBr} \)
\( \mathrm{H_2 + I_2 \rightleftharpoons 2HI} \)
Relative Reactivity in These Reactions
Trend: \( \mathrm{Cl_2 > Br_2 > I_2} \)
Explanation of the Reactivity Trend
- Halogens act as oxidising agents by gaining electrons from hydrogen
- Down the group, atomic radius increases
- There is increased shielding by inner electron shells
- The attraction between the nucleus and incoming electrons decreases
- Halogens gain electrons less readily down the group
As a result, chlorine reacts most vigorously with hydrogen, bromine reacts more slowly, and iodine reacts least readily and establishes an equilibrium.
The decreasing reactivity also reflects decreasing bond enthalpy and oxidising power of the halogens down Group 17.
Example
State the product formed when chlorine reacts with hydrogen.
▶️ Answer / Explanation
Hydrogen chloride, \( \mathrm{HCl} \).
Example
Explain why bromine reacts less readily with hydrogen than chlorine.
▶️ Answer / Explanation
Bromine atoms are larger and more shielded than chlorine atoms.
This reduces the attraction for electrons gained from hydrogen.
Therefore bromine is a weaker oxidising agent and reacts less readily with hydrogen.
Example
Explain why the reaction between hydrogen and iodine is reversible, while the reaction with chlorine is not.
▶️ Answer / Explanation
Iodine is a weak oxidising agent and gains electrons from hydrogen less readily.
The \( \mathrm{H–I} \) bond formed is relatively weak, so hydrogen iodide decomposes easily.
This leads to a reversible reaction and equilibrium mixture.
Chlorine is a much stronger oxidising agent and forms strong \( \mathrm{H–Cl} \) bonds, so the reaction goes to completion.
Thermal Stability of the Hydrogen Halides
The hydrogen halides, \( \mathrm{HX} \), show a clear trend in thermal stability down Group 17. Thermal stability refers to how readily a compound decomposes on heating.
Relative Thermal Stabilities
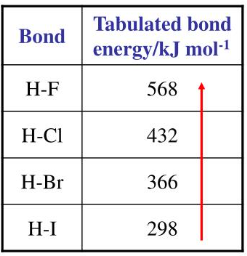
Trend: \( \mathrm{HCl > HBr > HI} \)
Hydrogen chloride is the most thermally stable, while hydrogen iodide is the least thermally stable.
Decomposition on Heating
On heating, hydrogen halides can decompose:
\( \mathrm{2HX \rightarrow H_2 + X_2} \)
Hydrogen iodide decomposes most readily, hydrogen bromide decomposes less readily, and hydrogen chloride is the most resistant to decomposition.
Explanation in Terms of Bond Strength
- Down Group 17, the size of the halogen atom increases
- The \( \mathrm{H–X} \) bond length increases
- The bonding pair of electrons is further from the nuclei
- There is greater shielding by inner electron shells
- The attraction between the nuclei and the bonding electrons decreases
As a result, the \( \mathrm{H–X} \) bond strength decreases from \( \mathrm{H–Cl} \) to \( \mathrm{H–I} \). Less energy is required to break weaker bonds, so thermal stability decreases down the group.
Hydrogen iodide has the weakest bond and therefore decomposes most easily when heated.
Example
State the order of thermal stability of the hydrogen halides \( \mathrm{HCl} \), \( \mathrm{HBr} \) and \( \mathrm{HI} \).
▶️ Answer / Explanation
\( \mathrm{HCl > HBr > HI} \)
Example
Explain why hydrogen bromide is less thermally stable than hydrogen chloride.
▶️ Answer / Explanation
Bromine atoms are larger than chlorine atoms, so the \( \mathrm{H–Br} \) bond is longer.
The bonding electrons are further from the nuclei and experience greater shielding.
This weakens the bond, so less energy is required to break it compared with the \( \mathrm{H–Cl} \) bond.
Example
Using bond strength ideas, explain why hydrogen iodide readily decomposes on heating.
▶️ Answer / Explanation
Iodine atoms are very large and have many inner electron shells.
This results in strong shielding and a long \( \mathrm{H–I} \) bond.
The electrostatic attraction between the nuclei and bonding electrons is weak.
Therefore only a small amount of energy is needed to break the \( \mathrm{H–I} \) bond, making hydrogen iodide thermally unstable.
