CIE AS/A Level Chemistry 11.3 Some reactions of the halide ions Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 11.3 Some reactions of the halide ions Study Notes – New Syllabus
CIE AS/A Level Chemistry 11.3 Some reactions of the halide ions Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe reducing ability trends
describe reactions with:
(a) Ag⁺(aq) followed by NH₃(aq)
(b) concentrated H₂SO₄
Relative Reactivity of Halide Ions as Reducing Agents
Halide ions, \( \mathrm{X^-} \), act as reducing agents because they donate electrons to other substances and are themselves oxidised to halogen molecules. Their reducing strength shows a clear trend down Group 17.
Reducing Agent Behaviour
A halide ion loses electrons:
\( \mathrm{2X^- \rightarrow X_2 + 2e^-} \)
Relative Reducing Strength
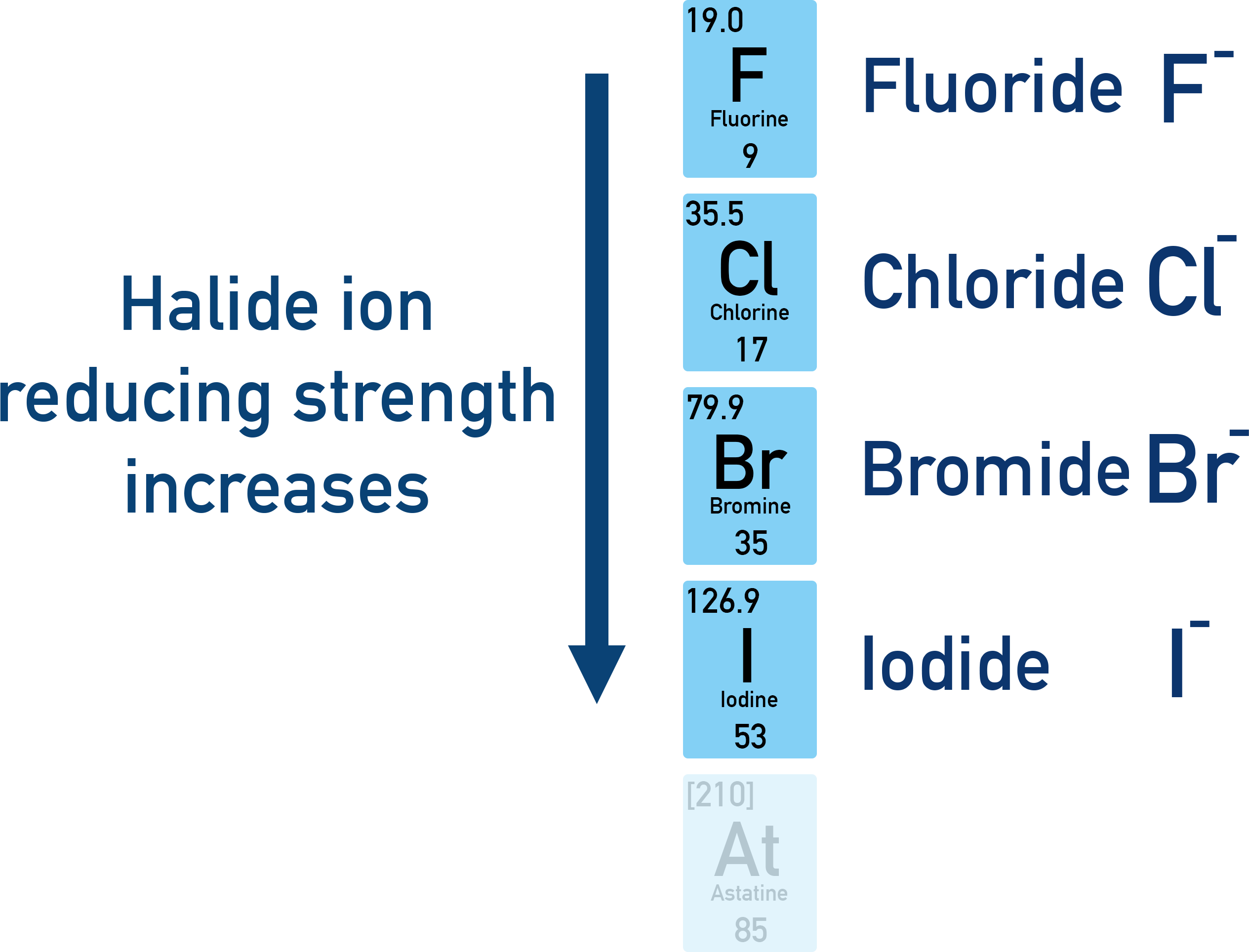
Trend: \( \mathrm{I^- > Br^- > Cl^-} \)
Iodide ions are the strongest reducing agents, while chloride ions are the weakest.
Explanation of the Trend
- Down Group 17, ionic radius increases
- The outer-shell electrons are further from the nucleus
- There is increased shielding by inner electron shells
- The attraction between the nucleus and outer electrons decreases
- Electrons are lost more easily down the group
As a result, iodide ions lose electrons most readily and are therefore the strongest reducing agents. Chloride ions hold onto their electrons most strongly and are the weakest reducing agents.
Evidence from Reactions
A stronger reducing agent can reduce a halogen molecule of a less reactive halide:
\( \mathrm{Cl_2 + 2I^- \rightarrow 2Cl^- + I_2} \)
Iodide ions reduce chlorine to chloride ions but chloride ions cannot reduce iodine.
Example
State which halide ion is the strongest reducing agent.
▶️ Answer / Explanation
Iodide, \( \mathrm{I^-} \).
Example
Explain why bromide ions are stronger reducing agents than chloride ions.
▶️ Answer / Explanation
Bromide ions are larger than chloride ions and have greater shielding.
This reduces the attraction between the nucleus and the outer electrons.
Electrons are lost more easily, so bromide ions are stronger reducing agents.
Example
Using ideas about atomic structure, explain why iodide ions readily reduce chlorine but chloride ions do not reduce iodine.
▶️ Answer / Explanation
Iodide ions are large and have many inner electron shells, giving strong shielding.
The outer electrons are weakly attracted to the nucleus and are easily lost.
This allows iodide ions to reduce chlorine by donating electrons.
Chloride ions are smaller with less shielding, so they hold electrons more strongly and cannot reduce iodine.
Reactions of Halide Ions
Halide ions show characteristic reactions that are used both for identification and for comparing their relative reducing strength. Two key reactions required at A level are with aqueous silver ions (followed by aqueous ammonia) and with concentrated sulfuric acid.
(a) Reactions with Aqueous Silver Ions, Followed by Aqueous Ammonia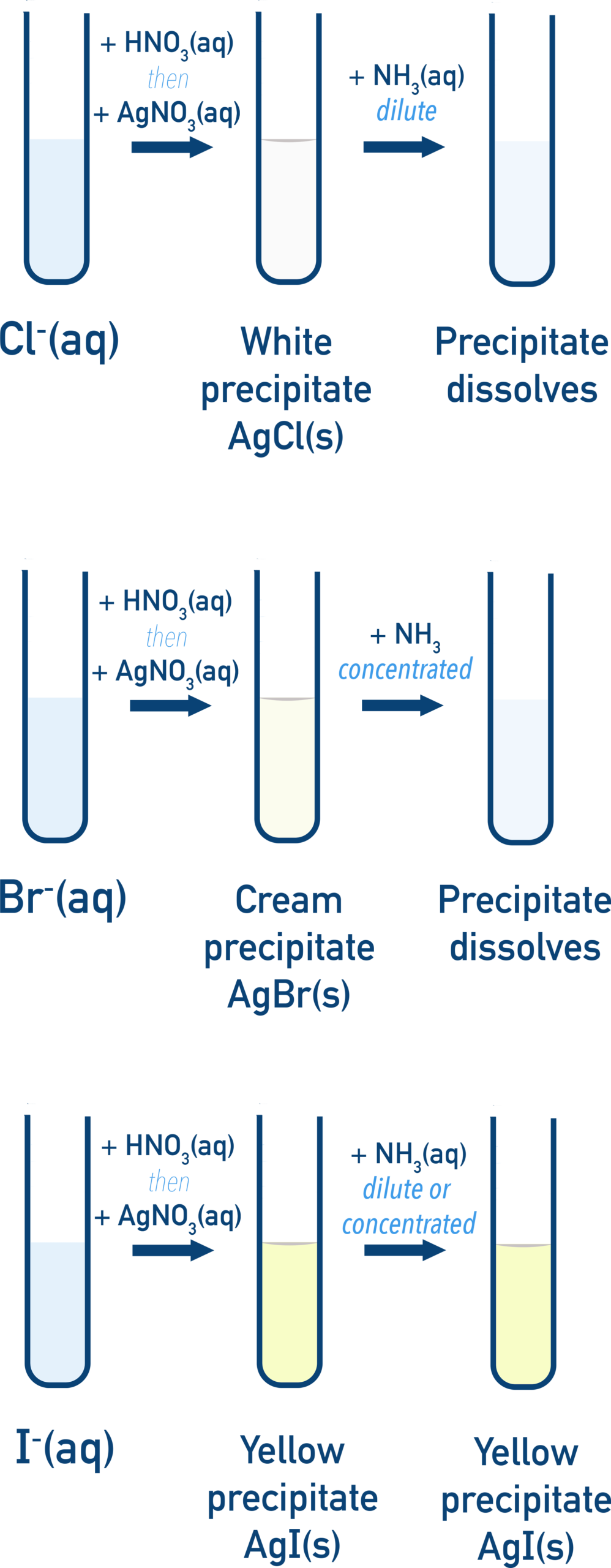
When aqueous silver ions are added to a solution containing halide ions, insoluble silver halide precipitates are formed.
General equation:
\( \mathrm{Ag^+ (aq) + X^- (aq) \rightarrow AgX (s)} \)
Observations and Explanations
- Chloride ions, \( \mathrm{Cl^-} \): white precipitate of silver chloride, \( \mathrm{AgCl} \)
- Bromide ions, \( \mathrm{Br^-} \): cream precipitate of silver bromide, \( \mathrm{AgBr} \)
- Iodide ions, \( \mathrm{I^-} \): yellow precipitate of silver iodide, \( \mathrm{AgI} \)
These precipitates form because silver halides have very low solubility in water.
Reaction with Aqueous Ammonia
The precipitates show different behaviour when aqueous ammonia is added:
- \( \mathrm{AgCl} \) dissolves in dilute ammonia
- \( \mathrm{AgBr} \) dissolves only in concentrated ammonia
- \( \mathrm{AgI} \) is insoluble in ammonia
Explanation: Down the group, the silver–halide bond becomes stronger and less easily broken. As a result, fewer silver ions are released to interact with ammonia, so solubility in ammonia decreases from \( \mathrm{AgCl} \) to \( \mathrm{AgI} \).
(b) Reactions with Concentrated Sulfuric Acid
Solid halide salts react with concentrated sulfuric acid. These reactions demonstrate the increasing reducing strength of the halide ions down Group 17.
Chloride Ions
Chloride ions undergo an acid–base reaction only:
\( \mathrm{NaCl (s) + H_2SO_4 (conc) \rightarrow NaHSO_4 (s) + HCl (g)} \)
Hydrogen chloride gas is produced; no redox reaction occurs because chloride ions are weak reducing agents.
Bromide Ions
Bromide ions initially undergo an acid–base reaction, followed by a redox reaction:
\( \mathrm{NaBr (s) + H_2SO_4 (conc) \rightarrow NaHSO_4 (s) + HBr (g)} \)
\( \mathrm{2HBr (g) + H_2SO_4 (conc) \rightarrow Br_2 (g) + SO_2 (g) + 2H_2O (l)} \)
Brown bromine vapour is observed. Bromide ions reduce sulfuric acid to sulfur dioxide.
Iodide Ions
Iodide ions are strong reducing agents and produce several redox products:
\( \mathrm{NaI (s) + H_2SO_4 (conc) \rightarrow NaHSO_4 (s) + HI (g)} \)
\( \mathrm{2HI (g) + H_2SO_4 (conc) \rightarrow I_2 (s) + SO_2 (g) + 2H_2O (l)} \)
\( \mathrm{6HI (g) + H_2SO_4 (conc) \rightarrow 3I_2 (s) + S (s) + 4H_2O (l)} \)
\( \mathrm{8HI (g) + H_2SO_4 (conc) \rightarrow 4I_2 (s) + H_2S (g) + 4H_2O (l)} \)
Purple iodine vapour and yellow sulfur solids may be seen, along with the smell of hydrogen sulfide. Iodide ions strongly reduce sulfuric acid.
Overall Explanation
Down Group 17, halide ions increase in size and shielding. Their outer electrons are held less strongly and are more easily lost. Therefore, reducing power increases from \( \mathrm{Cl^- < Br^- < I^-} \), explaining the increasing extent of redox reactions with concentrated sulfuric acid.
Example
A solution contains chloride ions. Describe what you would observe when aqueous silver nitrate is added, followed by dilute aqueous ammonia.
▶️ Answer / Explanation
A white precipitate of silver chloride, \( \mathrm{AgCl} \), forms when silver nitrate is added.
The precipitate dissolves in dilute aqueous ammonia.
This confirms the presence of chloride ions.
Example
Describe the reaction of solid potassium bromide with concentrated sulfuric acid. Include equations and observations.
▶️ Answer / Explanation
An acid–base reaction occurs first:
\( \mathrm{KBr (s) + H_2SO_4 (conc) \rightarrow KHSO_4 (s) + HBr (g)} \)
The hydrogen bromide then reduces sulfuric acid in a redox reaction:
\( \mathrm{2HBr (g) + H_2SO_4 (conc) \rightarrow Br_2 (g) + SO_2 (g) + 2H_2O (l)} \)
Brown bromine vapour is observed.
This shows that bromide ions act as reducing agents.
Example
A solid halide salt gives a yellow precipitate with aqueous silver nitrate that does not dissolve in aqueous ammonia. When the solid reacts with concentrated sulfuric acid, purple vapour and a rotten-egg smell are produced. Identify the halide ion and explain your answer.
▶️ Answer / Explanation
The halide ion is iodide, \( \mathrm{I^-} \).
A yellow precipitate that does not dissolve in ammonia indicates silver iodide, \( \mathrm{AgI} \).
With concentrated sulfuric acid, iodide ions act as strong reducing agents.
Purple vapour is iodine, \( \mathrm{I_2} \), and the rotten-egg smell is hydrogen sulfide, \( \mathrm{H_2S} \).
This confirms the presence of iodide ions.
