CIE AS/A Level Chemistry 11.4 The reactions of chlorine Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 11.4 The reactions of chlorine Study Notes – New Syllabus
CIE AS/A Level Chemistry 11.4 The reactions of chlorine Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe disproportionation reactions with cold and hot NaOH
explain chlorine use in water purification, including HOCl and ClO⁻ formation
Reaction of Chlorine with Aqueous Sodium Hydroxide
Chlorine reacts with aqueous sodium hydroxide in different ways depending on the temperature. In both cases, chlorine undergoes disproportionation, meaning it is simultaneously oxidised and reduced.
Definition: Disproportionation
A disproportionation reaction is one in which the same element is both oxidised and reduced.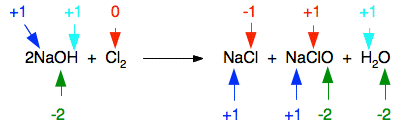
Reaction with Cold Aqueous Sodium Hydroxide
When chlorine reacts with cold, dilute aqueous sodium hydroxide:
\( \mathrm{Cl_2 (g) + 2NaOH (aq) \rightarrow NaCl (aq) + NaClO (aq) + H_2O (l)} \)
Oxidation Number Changes
- Chlorine in \( \mathrm{Cl_2} \) has oxidation number \( 0 \)
- Chlorine in \( \mathrm{NaCl} \) has oxidation number \( -1 \)
- Chlorine in \( \mathrm{NaClO} \) has oxidation number \( +1 \)
One chlorine atom is reduced from \( 0 \) to \( -1 \), and the other is oxidised from \( 0 \) to \( +1 \). This confirms the reaction is a disproportionation reaction.
Reaction with Hot Aqueous Sodium Hydroxide
When chlorine reacts with hot, concentrated aqueous sodium hydroxide:

\( \mathrm{3Cl_2 (g) + 6NaOH (aq) \rightarrow 5NaCl (aq) + NaClO_3 (aq) + 3H_2O (l)} \)
Oxidation Number Changes
- Chlorine in \( \mathrm{Cl_2} \): oxidation number \( 0 \)
- Chlorine in \( \mathrm{NaCl} \): oxidation number \( -1 \)
- Chlorine in \( \mathrm{NaClO_3} \): oxidation number \( +5 \)
Some chlorine atoms are reduced from \( 0 \) to \( -1 \), while others are oxidised from \( 0 \) to \( +5 \). This is also a disproportionation reaction.
Comparison of Cold and Hot Reactions
- Cold conditions produce chloride and chlorate(I) ions
- Hot conditions produce chloride and chlorate(V) ions
- Higher temperature leads to a greater extent of oxidation of chlorine
Example
State the products formed when chlorine reacts with cold aqueous sodium hydroxide.
▶️ Answer / Explanation
Sodium chloride, sodium chlorate(I) and water.
Example
Explain why the reaction between chlorine and cold aqueous sodium hydroxide is described as disproportionation.
▶️ Answer / Explanation
Chlorine starts with oxidation number \( 0 \).
Some chlorine is reduced to chloride with oxidation number \( -1 \).
Some chlorine is oxidised to chlorate(I) with oxidation number \( +1 \).
Because chlorine is both oxidised and reduced, the reaction is disproportionation.
Example
Describe and interpret, in terms of oxidation numbers, the reaction of chlorine with hot aqueous sodium hydroxide.
▶️ Answer / Explanation
Chlorine reacts with hot concentrated sodium hydroxide to form chloride ions and chlorate(V) ions.
Chlorine is reduced from oxidation number \( 0 \) to \( -1 \) in chloride ions.
Chlorine is oxidised from oxidation number \( 0 \) to \( +5 \) in chlorate(V) ions.
This shows the reaction is a disproportionation reaction.
Use of Chlorine in Water Purification
Chlorine is widely used to disinfect drinking water. Its effectiveness is due to the formation of reactive chlorine-containing species in water that kill bacteria and other microorganisms.
Reaction of Chlorine with Water
When chlorine is added to water, it reacts to form hydrochloric acid and hypochlorous acid: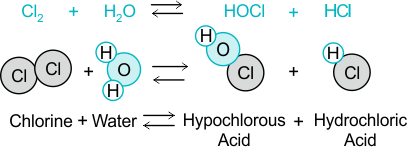
\( \mathrm{Cl_2 (g) + H_2O (l) \rightleftharpoons HCl (aq) + HOCl (aq)} \)
Hypochlorous acid, \( \mathrm{HOCl} \), is the main active disinfecting species.
Formation of Hypochlorite Ions
Hypochlorous acid partially dissociates in water:
\( \mathrm{HOCl (aq) \rightleftharpoons H^+ (aq) + ClO^- (aq)} \)
Both \( \mathrm{HOCl} \) and \( \mathrm{ClO^-} \) act as oxidising agents and are responsible for killing bacteria.
How \( \mathrm{HOCl} \) and \( \mathrm{ClO^-} \) Kill Bacteria

- \( \mathrm{HOCl} \) is neutral and can easily penetrate bacterial cell walls
- Inside the cell, it acts as a strong oxidising agent
- It oxidises essential enzymes and proteins
- This disrupts metabolic reactions and damages cell membranes
- The bacteria are killed
The hypochlorite ion, \( \mathrm{ClO^-} \), also acts as an oxidising agent, but is less effective than \( \mathrm{HOCl} \) because it is negatively charged and penetrates cell walls less easily.
Overall Interpretation
Chlorine is effective in water purification because it forms \( \mathrm{HOCl} \) and \( \mathrm{ClO^-} \), both of which oxidise and destroy bacteria. The presence of an equilibrium ensures a continuous supply of the active disinfecting species.
Example
State the active species formed when chlorine dissolves in water during purification.
▶️ Answer / Explanation
Hypochlorous acid, \( \mathrm{HOCl} \), and hypochlorite ions, \( \mathrm{ClO^-} \).
Example
Write an equation to show how hypochlorous acid is formed when chlorine is added to water.
▶️ Answer / Explanation
\( \mathrm{Cl_2 + H_2O \rightleftharpoons HCl + HOCl} \)
Example
Explain, using equations, how chlorine added to water kills bacteria.
▶️ Answer / Explanation
Chlorine reacts with water to form hypochlorous acid:
\( \mathrm{Cl_2 + H_2O \rightleftharpoons HCl + HOCl} \)
Hypochlorous acid dissociates to form hypochlorite ions:
\( \mathrm{HOCl \rightleftharpoons H^+ + ClO^-} \)
\( \mathrm{HOCl} \) and \( \mathrm{ClO^-} \) are oxidising agents that oxidise enzymes and proteins in bacteria, killing them.
