CIE AS/A Level Chemistry 12.1 Nitrogen and sulfur Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 12.1 Nitrogen and sulfur Study Notes – New Syllabus
CIE AS/A Level Chemistry 12.1 Nitrogen and sulfur Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
explain the low reactivity of nitrogen due to strong triple bond and non-polarity
describe:
(a) basicity of ammonia
(b) formation and structure of NH₄⁺
(c) displacement of ammonia from ammonium saltsdescribe sources of nitrogen oxides and their catalytic removal
understand formation of peroxyacetyl nitrate (PAN) in photochemical smog
describe the role of NO and NO₂ in acid rain formation
Lack of Reactivity of Nitrogen
Nitrogen is a Group 15 element that exists as a simple diatomic molecule, \( \mathrm{N_2} \), under normal conditions. Despite nitrogen being abundant in the atmosphere, it is largely unreactive. This lack of reactivity can be explained by the strength of the triple bond and the non-polar nature of the molecule.
Structure of the Nitrogen Molecule
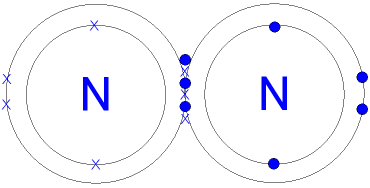
\( \mathrm{N \equiv N} \)
The two nitrogen atoms are joined by a triple covalent bond, consisting of one sigma bond and two pi bonds.
Triple Bond Strength
- The \( \mathrm{N \equiv N} \) triple bond has a very high bond enthalpy
- This is due to strong overlap of small nitrogen \( 2p \) orbitals
- The nuclei are very close together, producing a strong electrostatic attraction
- A large amount of energy is required to break the triple bond
Because breaking the \( \mathrm{N \equiv N} \) bond requires so much energy, most reactions involving nitrogen have a very high activation energy. As a result, nitrogen reacts only under extreme conditions, such as high temperature, high pressure, or in the presence of a catalyst.
Lack of Polarity
The nitrogen molecule is non-polar:

- Both atoms in \( \mathrm{N_2} \) are identical
- The electronegativity difference between the atoms is zero
- There is no permanent dipole
As a result, nitrogen experiences only weak instantaneous dipole–induced dipole forces. It does not readily interact with polar molecules or ions, further reducing its reactivity.
Overall Explanation
Nitrogen is unreactive because the \( \mathrm{N \equiv N} \) triple bond is extremely strong and requires a large amount of energy to break. In addition, the molecule is non-polar and does not strongly attract reactants. These factors together explain why nitrogen shows very low reactivity under normal conditions.
Example
State one reason why nitrogen is unreactive.
▶️ Answer / Explanation
Nitrogen contains a very strong triple bond that requires a large amount of energy to break.
Example
Explain why nitrogen molecules have a high activation energy for reaction.
▶️ Answer / Explanation
The \( \mathrm{N \equiv N} \) triple bond is very strong due to effective orbital overlap.
A large amount of energy is needed to break this bond before reactions can occur.
Example
Explain the lack of reactivity of nitrogen with reference to bond strength and polarity.
▶️ Answer / Explanation
Nitrogen molecules contain a strong \( \mathrm{N \equiv N} \) triple bond with very high bond enthalpy.
Breaking this bond requires a large activation energy.
In addition, \( \mathrm{N_2} \) is non-polar because the atoms are identical and have equal electronegativity.
As a result, nitrogen has weak intermolecular attractions and does not readily interact with other substances.
Reactions and Acid–Base Behaviour of Ammonia and Ammonium Compounds
Ammonia and ammonium compounds are central to Brønsted–Lowry acid–base theory. Their reactions can be explained using proton transfer, lone pairs, and changes in structure.
(a) Basicity of Ammonia (Brønsted–Lowry Theory)
According to the Brønsted–Lowry theory:
A base is a proton (\( \mathrm{H^+} \)) acceptor.
Ammonia, \( \mathrm{NH_3} \), is a weak base because it can accept a proton using the lone pair of electrons on the nitrogen atom.
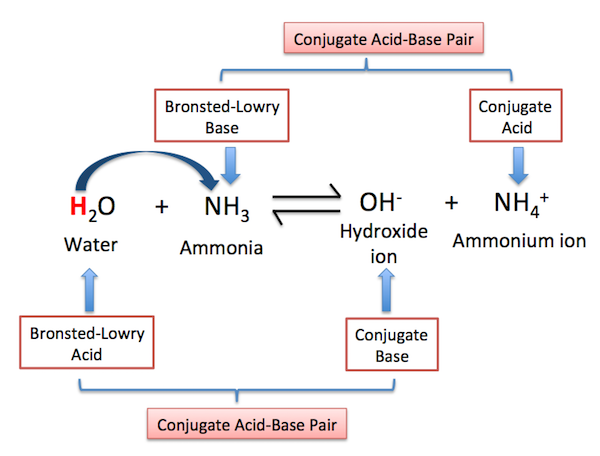
\( \mathrm{NH_3 (aq) + H_2O (l) \rightleftharpoons NH_4^+ (aq) + OH^- (aq)} \)
Explanation
- Nitrogen has a lone pair of electrons
- This lone pair can accept a proton from water
- Ammonia acts as a Brønsted–Lowry base
- The equilibrium lies to the left, so ammonia is a weak base
(b) Structure and Formation of the Ammonium Ion
When ammonia accepts a proton, it forms the ammonium ion, \( \mathrm{NH_4^+} \).

Structure of the Ammonium Ion
- Tetrahedral shape
- Bond angle of approximately \( 109.5^\circ \)
- Four identical \( \mathrm{N–H} \) bonds
- No lone pair on nitrogen
Formation by Acid–Base Reaction
Ammonium ions form when ammonia reacts with an acid:
\( \mathrm{NH_3 (aq) + H^+ (aq) \rightarrow NH_4^+ (aq)} \)
The proton is accepted by the lone pair on nitrogen, forming a dative (coordinate) covalent bond. Once formed, all four \( \mathrm{N–H} \) bonds are identical.
(c) Displacement of Ammonia from Ammonium Salts
Ammonia can be displaced from ammonium salts by reacting them with a strong base. This is an acid–base reaction.
Example: Ammonium Chloride and Sodium Hydroxide
\( \mathrm{NH_4Cl (s) + NaOH (aq) \rightarrow NH_3 (g) + NaCl (aq) + H_2O (l)} \)
Ionic Equation
\( \mathrm{NH_4^+ (aq) + OH^- (aq) \rightarrow NH_3 (g) + H_2O (l)} \)
Explanation
- The ammonium ion acts as a Brønsted–Lowry acid
- It donates a proton to hydroxide ions
- Ammonia gas is released
- This reaction is used as a test for ammonium ions
Example
Explain why ammonia is a Brønsted–Lowry base.
▶️ Answer / Explanation
Ammonia has a lone pair of electrons that can accept a proton.
Example
Describe the structure of the ammonium ion and explain how it is formed.
▶️ Answer / Explanation
The ammonium ion has a tetrahedral shape with bond angles of \( 109.5^\circ \).
It forms when ammonia accepts a proton using its lone pair, creating a dative covalent bond.
Example
Explain how ammonia is displaced from ammonium salts by sodium hydroxide.
▶️ Answer / Explanation
Ammonium ions donate a proton to hydroxide ions.
This forms water and ammonia gas.
\( \mathrm{NH_4^+ + OH^- \rightarrow NH_3 + H_2O} \)
This is an acid–base reaction.
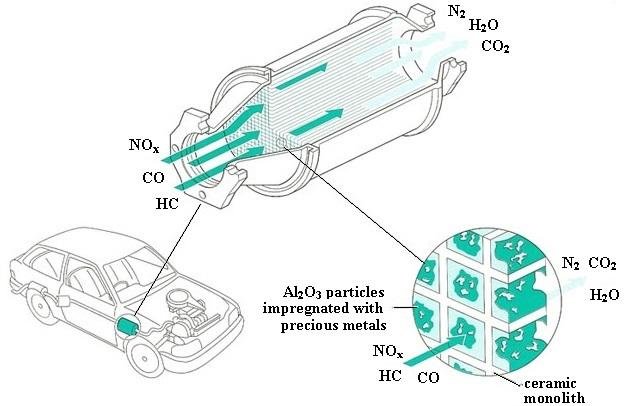
Oxides of Nitrogen: Occurrence and Catalytic Removal
Oxides of nitrogen, commonly referred to as \( \mathrm{NO_x} \), mainly include nitrogen monoxide, \( \mathrm{NO} \), and nitrogen dioxide, \( \mathrm{NO_2} \). They are important atmospheric pollutants formed naturally and by human activity, especially in internal combustion engines.
Natural Occurrence of Oxides of Nitrogen
Oxides of nitrogen are formed naturally in the atmosphere at very high temperatures.
- Lightning: The high temperatures produced during lightning allow nitrogen and oxygen in air to react
\( \mathrm{N_2 (g) + O_2 (g) \rightarrow 2NO (g)} \)
The nitrogen monoxide formed then reacts with oxygen:
\( \mathrm{2NO (g) + O_2 (g) \rightarrow 2NO_2 (g)} \)
These oxides dissolve in rainwater to form dilute nitric acid, contributing naturally to soil nitrates.
Man-Made Occurrence of Oxides of Nitrogen
Large quantities of oxides of nitrogen are produced by human activities involving high-temperature combustion.
- Internal combustion engines: High temperatures inside car engines allow nitrogen and oxygen from air to react

\( \mathrm{N_2 (g) + O_2 (g) \rightarrow 2NO (g)} \)
The nitrogen monoxide formed is further oxidised in the exhaust:
\( \mathrm{2NO (g) + O_2 (g) \rightarrow 2NO_2 (g)} \)
Nitrogen dioxide contributes to photochemical smog, acid rain and respiratory problems.
Catalytic Removal of \( \mathrm{NO_x} \) from Exhaust Gases
Oxides of nitrogen are removed from car exhausts using catalytic converters. These contain catalysts such as platinum, palladium and rhodium.
Role of the Catalyst
- Provides a surface for reactions to occur
- Adsorbs reactant molecules, lowering activation energy

- Allows redox reactions to proceed more rapidly
Key Reactions in a Catalytic Converter
Nitrogen monoxide is reduced to nitrogen:
\( \mathrm{2NO (g) \rightarrow N_2 (g) + O_2 (g)} \)
More commonly, nitrogen monoxide reacts with carbon monoxide:
\( \mathrm{2NO (g) + 2CO (g) \rightarrow N_2 (g) + 2CO_2 (g)} \)
In these reactions, nitrogen monoxide is reduced, while carbon monoxide is oxidised.
Overall Importance
Catalytic converters significantly reduce emissions of nitrogen oxides from vehicles. By converting harmful \( \mathrm{NO_x} \) gases into nitrogen and carbon dioxide, they help limit air pollution, acid rain and health risks.
Example
State one natural source of oxides of nitrogen.
▶️ Answer / Explanation
Lightning.
Example
Explain why oxides of nitrogen are formed in car engines.
▶️ Answer / Explanation
The very high temperatures inside car engines provide enough energy to break the strong \( \mathrm{N \equiv N} \) bond.
This allows nitrogen and oxygen from air to react, forming nitrogen monoxide.
Example
Describe how a catalytic converter removes nitrogen monoxide from exhaust gases.
▶️ Answer / Explanation
Nitrogen monoxide is adsorbed onto the catalyst surface.
It is reduced to nitrogen gas.
Carbon monoxide is oxidised to carbon dioxide.
\( \mathrm{2NO + 2CO \rightarrow N_2 + 2CO_2} \)
Formation of Peroxyacetyl Nitrate (PAN) in Photochemical Smog
In polluted atmospheres, particularly in strong sunlight, oxides of nitrogen (\( \mathrm{NO} \) and \( \mathrm{NO_2} \)) can react with unburned hydrocarbons from vehicle exhausts. These reactions lead to the formation of peroxyacetyl nitrate (PAN), a major component of photochemical smog.
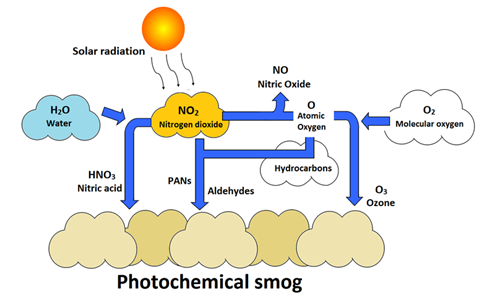
Sources of Reactants
- \( \mathrm{NO} \) and \( \mathrm{NO_2} \) are produced in internal combustion engines at high temperatures
- Unburned hydrocarbons are released from incomplete combustion of petrol and diesel fuels
- Sunlight provides the energy needed to initiate reactions
Role of Sunlight
Sunlight causes nitrogen dioxide to photodissociate:
\( \mathrm{NO_2 (g) \xrightarrow{UV\ light} NO (g) + O \cdot (g)} \)
The oxygen radical formed leads to further radical reactions in the atmosphere.
Reaction with Unburned Hydrocarbons
Hydrocarbons react with oxygen and radicals to form peroxyacyl radicals, such as the peroxyacetyl radical:
\( \mathrm{CH_3CO \cdot + O_2 \rightarrow CH_3COO_2 \cdot} \)
These radicals then react with nitrogen dioxide to form peroxyacetyl nitrate:
\( \mathrm{CH_3COO_2 \cdot + NO_2 \rightleftharpoons CH_3COOONO_2} \)
The product, \( \mathrm{CH_3COOONO_2} \), is peroxyacetyl nitrate (PAN).
PAN and Photochemical Smog
- PAN is a secondary pollutant (not emitted directly)
- It is a strong eye irritant and respiratory irritant
- It damages plant tissue and reduces crop yields
- It is stable at lower temperatures, allowing it to travel long distances
PAN is therefore an important and harmful component of photochemical smog, which forms in sunny, polluted urban environments.
Overall Understanding
Atmospheric \( \mathrm{NO} \) and \( \mathrm{NO_2} \), produced mainly by vehicles, react with unburned hydrocarbons in the presence of sunlight. These reactions form peroxyacetyl nitrate (PAN), contributing to photochemical smog and its associated health and environmental problems.
Example
Name the pollutant formed when nitrogen dioxide reacts with hydrocarbons in sunlight.
▶️ Answer / Explanation
Peroxyacetyl nitrate (PAN).
Example
Explain why PAN is described as a secondary pollutant.
▶️ Answer / Explanation
PAN is not emitted directly into the atmosphere.
It is formed by reactions between nitrogen dioxide and hydrocarbons in sunlight.
Example
Describe how oxides of nitrogen contribute to the formation of photochemical smog.
▶️ Answer / Explanation
Nitrogen dioxide is formed in vehicle engines and released into the atmosphere.
Sunlight causes nitrogen dioxide to form radicals.
These radicals react with unburned hydrocarbons to form peroxyacetyl radicals.
Reaction with nitrogen dioxide produces peroxyacetyl nitrate, PAN.
PAN is a toxic component of photochemical smog.
Role of \( \mathrm{NO} \) and \( \mathrm{NO_2} \) in the Formation of Acid Rain
Atmospheric oxides of nitrogen, mainly nitrogen monoxide (\( \mathrm{NO} \)) and nitrogen dioxide (\( \mathrm{NO_2} \)), contribute to acid rain in two ways:
- directly, by forming nitric acid
- indirectly, by acting as catalysts in the oxidation of sulfur dioxide to sulfuric acid
(a) Direct Contribution: Formation of Nitric Acid
Oxides of nitrogen are produced in vehicle engines and power stations at high temperatures. In the atmosphere, they react further with oxygen and water to form nitric acid.
Step 1: Oxidation of Nitrogen Monoxide
\( \mathrm{2NO (g) + O_2 (g) \rightarrow 2NO_2 (g)} \)
Step 2: Reaction of Nitrogen Dioxide with Water
\( \mathrm{3NO_2 (g) + H_2O (l) \rightarrow 2HNO_3 (aq) + NO (g)} \)
Nitric acid formed dissolves in rainwater, lowering its pH and contributing to acid rain.
(b) Catalytic Role in the Oxidation of Sulfur Dioxide
Sulfur dioxide, \( \mathrm{SO_2} \), is released mainly from burning sulfur-containing fossil fuels. Oxides of nitrogen catalyse its oxidation to sulfuric acid in the atmosphere.
Key Catalytic Steps
Nitrogen dioxide oxidises sulfur dioxide:
\( \mathrm{SO_2 (g) + NO_2 (g) \rightarrow SO_3 (g) + NO (g)} \)
The nitrogen monoxide produced is re-oxidised by oxygen:
\( \mathrm{2NO (g) + O_2 (g) \rightarrow 2NO_2 (g)} \)
This regenerates \( \mathrm{NO_2} \), allowing it to oxidise more sulfur dioxide. Because \( \mathrm{NO_2} \) is regenerated, it acts as a catalyst.
Formation of Sulfuric Acid
Sulfur trioxide formed then reacts with water:
\( \mathrm{SO_3 (g) + H_2O (l) \rightarrow H_2SO_4 (aq)} \)
Sulfuric acid dissolves in rainwater and is a major contributor to acid rain.
Overall Interpretation
Oxides of nitrogen contribute to acid rain directly by forming nitric acid and indirectly by catalysing the oxidation of sulfur dioxide to sulfuric acid. Their catalytic role increases the rate of sulfuric acid formation, making \( \mathrm{NO} \) and \( \mathrm{NO_2} \) particularly important atmospheric pollutants.
Example
Name the acid formed directly from nitrogen dioxide in acid rain.
▶️ Answer / Explanation
Nitric acid, \( \mathrm{HNO_3} \).
Example
Explain how nitrogen dioxide acts as a catalyst in the oxidation of sulfur dioxide.
▶️ Answer / Explanation
Nitrogen dioxide oxidises sulfur dioxide to sulfur trioxide.
It is reduced to nitrogen monoxide.
Nitrogen monoxide is re-oxidised by oxygen to nitrogen dioxide.
Because nitrogen dioxide is regenerated, it acts as a catalyst.
Example
Describe the role of oxides of nitrogen in the formation of acid rain.
▶️ Answer / Explanation
Nitrogen dioxide reacts with water to form nitric acid, contributing directly to acid rain.
Nitrogen dioxide also catalyses the oxidation of sulfur dioxide to sulfur trioxide.
Sulfur trioxide reacts with water to form sulfuric acid.
Because nitrogen dioxide is regenerated, it acts as a catalyst.