CIE AS/A Level Chemistry 13.1 Formulae, functional groups and the naming of organic compounds Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 13.1 Formulae, functional groups and the naming of organic compounds Study Notes – New Syllabus
CIE AS/A Level Chemistry 13.1 Formulae, functional groups and the naming of organic compounds Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
define the term hydrocarbon as a compound made up of C and H atoms only
understand that alkanes are simple hydrocarbons with no functional group
understand that the compounds in the table on pages 29 and 30 contain a functional group which
dictates their physical and chemical propertiesinterpret and use the general, structural, displayed and skeletal formulas of the classes of compound
stated in the table on pages 29 and 30understand and use systematic nomenclature of simple aliphatic organic molecules with functional
groups detailed in the table on pages 29 and 30, up to six carbon atoms
(six plus six for esters, straight chains only for esters and nitriles)deduce the molecular and/or empirical formula of a compound, given its structural, displayed or
skeletal formula
Hydrocarbons
Hydrocarbons are the simplest type of organic compounds and form the foundation of organic chemistry. All other organic molecules can be considered as derivatives of hydrocarbons.
Definition
A hydrocarbon is a compound made up of carbon (C) and hydrogen (H) atoms only.
Key Features
- Contain only carbon and hydrogen atoms
- Do not contain oxygen, nitrogen, sulfur, halogens, or metals
- Carbon atoms form a chain or ring structure
- Hydrogen atoms are bonded to carbon atoms
Types of Hydrocarbons
![]()
- Alkanes – saturated hydrocarbons (single C–C bonds only)
- Alkenes – unsaturated hydrocarbons containing C=C double bonds
- Alkynes – unsaturated hydrocarbons containing C≡C triple bonds
- Aromatic hydrocarbons – contain benzene rings
At A level, it is essential that the definition clearly states only carbon and hydrogen atoms. Mentioning anything else (e.g. “mainly carbon and hydrogen”) would lose marks.
Example
State the definition of a hydrocarbon.
▶️ Answer / Explanation
A hydrocarbon is a compound made up of carbon and hydrogen atoms only.
Example
Ethene has the molecular formula \( \mathrm{C_2H_4} \). Explain why ethene is classified as a hydrocarbon.
▶️ Answer / Explanation
Ethene contains only carbon and hydrogen atoms.
No other elements are present, so it is a hydrocarbon.
Example
Compound A has the molecular formula \( \mathrm{C_3H_7Cl} \). Compound B has the molecular formula \( \mathrm{C_3H_8} \). Identify which compound is a hydrocarbon and justify your answer.
▶️ Answer / Explanation
Compound B, \( \mathrm{C_3H_8} \), is a hydrocarbon.
It contains only carbon and hydrogen atoms.
Compound A is not a hydrocarbon because it contains chlorine.
Alkanes
Alkanes are a class of simple hydrocarbons. They are the simplest members of the hydrocarbon family and form the starting point for the study of organic chemistry.
Definition
An alkane is a saturated hydrocarbon that contains only carbon and hydrogen atoms and has no functional group.
Key Features![]()
- Contain only carbon and hydrogen atoms
- Have no functional group
- Are saturated, with only single C–C bonds
- General formula \( \mathrm{C_{n}H_{2n+2}} \)
- Relatively unreactive compared to other organic compounds
At A level, it is important to state clearly that alkanes have no functional group. Any suggestion of a functional group would be incorrect.
Example
State one reason why alkanes are described as simple hydrocarbons.
▶️ Answer / Explanation
Alkanes contain only carbon and hydrogen atoms.
They have no functional group.
Example
Propane has the molecular formula \( \mathrm{C_3H_8} \). Explain why propane has no functional group.
▶️ Answer / Explanation
Propane contains only carbon–carbon single bonds and carbon–hydrogen bonds.
There are no specific reactive groups present, so it has no functional group.
Example
Compound A has the formula \( \mathrm{C_4H_{10}} \). Compound B has the formula \( \mathrm{C_4H_9OH} \). Identify which compound is an alkane and justify your answer.
▶️ Answer / Explanation
Compound A, \( \mathrm{C_4H_{10}} \), is an alkane.
It contains only carbon and hydrogen atoms and has no functional group.
Compound B is not an alkane because it contains an –OH functional group.
Functional Groups and Their Importance
The compounds shown in the table belong to different homologous series. Each of these compounds contains a functional group.
| Homologous series | Name of functional group | Structural formula of functional group | Displayed formula (example) | Skeletal formula (example) | Example name |
|---|---|---|---|---|---|
| Alkene | C=C bond |  |  | 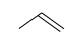 | propene |
| Halogenoalkane | Halogen |  |  | 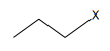 | 1-chloropropane |
| Alcohol | Hydroxyl |  | propan-1-ol | ||
| Aldehyde | Carbonyl |  |  | propanal | |
| Ketone | Carbonyl |  |  |  | propanone |
| Carboxylic acid | Carboxyl |  |  |  | propanoic acid |
| Ester | Ester | 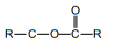 |  |  | ethyl ethanoate |
| Amine (primary) | Amine | 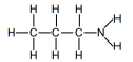 |  | propylamine | |
| Nitrile | Nitrile |  | 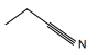 | propanenitrile |
Definition
A functional group is an atom or group of atoms that is responsible for the characteristic physical and chemical properties of an organic compound.
Key Points
- All compounds in the table contain a functional group
- The functional group determines the compound’s reactivity
- The functional group affects physical properties, such as boiling point and solubility
- Compounds with the same functional group show similar chemical reactions
- Different functional groups give rise to different homologous series
At A level, it is essential to understand that it is the functional group, not the carbon chain length alone, that dictates the physical and chemical properties of a compound.
Example
State what determines the chemical properties of an organic compound.
▶️ Answer / Explanation
The chemical properties are determined by the functional group present.
Example
Propanol and propanoic acid have similar chain lengths. Explain why their chemical properties are different.
▶️ Answer / Explanation
Propanol contains an –OH functional group, while propanoic acid contains a –COOH functional group.
Different functional groups result in different chemical properties.
Example
Ethanoic acid and ethyl ethanoate both contain two carbon atoms. Explain why they have different physical and chemical properties.
▶️ Answer / Explanation
Ethanoic acid contains a carboxyl functional group, while ethyl ethanoate contains an ester functional group.
The different functional groups lead to different intermolecular forces and reactivity.
Interpreting Organic Formulae
Organic compounds can be represented in several different ways. At A level, you must be able to interpret and use general, structural, displayed, and skeletal formulae for the classes of compounds listed in the table.
Types of Formula

General formula shows the relationship between the number of carbon and hydrogen atoms in a homologous series.
Structural formula shows how atoms are arranged and how the functional group is attached, using condensed notation.
Displayed formula shows all atoms and all covalent bonds explicitly.
Skeletal formula shows the carbon framework only; carbon atoms are implied at the ends and bends of lines, and hydrogen atoms bonded to carbon are omitted.
Key Points
- Compounds in the same homologous series share the same general formula
- The functional group must always be shown in structural, displayed, and skeletal formulae
- Skeletal formulae do not show C–H bonds
- Heteroatoms (e.g. O, N, halogens) and bonds to them are always shown
At A level, you must be able to move confidently between all four types of formula.
Example
State the general formula of alkanes.
▶️ Answer / Explanation
The general formula of alkanes is \( \mathrm{C_{n}H_{2n+2}} \).
Example
The skeletal formula of a compound shows a three-carbon chain ending in –OH. Name the compound.
▶️ Answer / Explanation
The compound is propan-1-ol.
The –OH functional group identifies it as an alcohol.
Example
A compound has the general formula \( \mathrm{C_{n}H_{2n}} \) and contains a C=C bond. Draw its skeletal formula for the three-carbon member and state its name.
▶️ Answer / Explanation
The three-carbon member is propene.
Its skeletal formula is a three-carbon zig-zag chain with one C=C double bond.
The general formula and C=C bond identify it as an alkene.
Systematic Nomenclature of Aliphatic Organic Molecules
At A level, you must be able to understand and use systematic nomenclature to name simple aliphatic organic molecules containing the functional groups listed in the table.
This applies to molecules with up to six carbon atoms. For esters, up to six carbon atoms in the alcohol part and six in the acid part may be used. For esters and nitriles, only straight-chain molecules are considered.
Key Principles of Systematic Naming

- Identify the longest carbon chain containing the functional group
- Identify the functional group to determine the suffix
- Number the carbon chain to give the functional group the lowest possible number
- Use prefixes (meth-, eth-, prop-, but-, pent-, hex-) to indicate chain length
- Only aliphatic (non-aromatic) compounds are included
Special Naming Rules

- Alcohols use the suffix -ol (e.g. propan-1-ol)
- Alkenes use the suffix -ene and require a position number
- Aldehydes use the suffix -al (carbonyl is always on carbon 1)
- Ketones use the suffix -one with a position number
- Carboxylic acids use the suffix -oic acid
- Esters are named as alkyl alkanoates
- Nitriles use the suffix -nitrile and include the carbon of the C≡N group in the chain length

At A level, all names must be fully systematic. Common or trivial names are not accepted unless explicitly stated.
Example
Name the straight-chain alcohol with the structural formula \( \mathrm{CH_3CH_2OH} \).
▶️ Answer / Explanation
The longest chain has two carbon atoms, giving the prefix eth-.
The functional group is –OH, so the suffix is -ol.
The compound is named ethanol.
Example
Give the systematic name of the ketone with molecular formula \( \mathrm{C_5H_{10}O} \) and the carbonyl group on carbon 2.
▶️ Answer / Explanation
The longest carbon chain contains five carbon atoms, giving the prefix pent-.
The functional group is a ketone, so the suffix is -one.
The carbonyl group is on carbon 2, giving the name pentan-2-one.
Example
An ester is formed from a straight-chain alcohol containing three carbon atoms and a straight-chain carboxylic acid containing four carbon atoms. Name the ester.
▶️ Answer / Explanation
The alcohol forms the alkyl part, which is propyl.
The carboxylic acid forms the alkanoate part, which is butanoate.
The ester is named propyl butanoate.
Deducing Molecular and Empirical Formulae
At A level, you must be able to deduce the molecular and/or empirical formula of an organic compound when given its structural, displayed, or skeletal formula.

Definitions
The molecular formula shows the actual number of each type of atom present in one molecule of a compound.
The empirical formula shows the simplest whole-number ratio of atoms in the compound.
Method
- Count all atoms shown or implied in the formula
- Remember that skeletal formulae do not show C or H atoms explicitly
- Carbon atoms are present at each end and bend of a skeletal formula
- Hydrogen atoms are added to each carbon to give a total of four bonds
- Once the molecular formula is found, simplify it to obtain the empirical formula if required
For skeletal formulae, always add hydrogen atoms last after counting all heteroatoms and multiple bonds.
Example
A compound has the structural formula \( \mathrm{CH_3CH_3} \). State its molecular formula.
▶️ Answer / Explanation
The molecule contains two carbon atoms and six hydrogen atoms.
The molecular formula is \( \mathrm{C_2H_6} \).
Example
A displayed formula shows three carbon atoms in a chain with a C=C double bond between the first and second carbon atoms. Deduce the molecular formula.
▶️ Answer / Explanation
The molecule contains three carbon atoms.
The presence of a C=C double bond reduces the number of hydrogen atoms.
Adding hydrogens to give four bonds per carbon gives eight hydrogen atoms.
The molecular formula is \( \mathrm{C_3H_6} \).
Example
A skeletal formula shows a four-carbon chain ending in a –COOH group. Deduce the molecular and empirical formulae.
▶️ Answer / Explanation
The skeletal formula represents a carboxylic acid with four carbon atoms.
Counting atoms gives the molecular formula \( \mathrm{C_4H_8O_2} \).
Dividing all subscripts by 2 gives the empirical formula \( \mathrm{C_2H_4O} \).
