CIE AS/A Level Chemistry 13.2 Characteristic organic reactions Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 13.2 Characteristic organic reactions Study Notes – New Syllabus
CIE AS/A Level Chemistry 13.2 Characteristic organic reactions Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
interpret and use the following terminology associated with types of organic compounds and reactions:
(a) homologous series
(b) saturated and unsaturated
(c) homolytic and heterolytic fission
(d) free radical, initiation, propagation, termination
(e) nucleophile, electrophile, nucleophilic, electrophilic
(f) addition, substitution, elimination, hydrolysis, condensation
(g) oxidation and reduction(in equations for organic redox reactions, the symbol [O] can be used to represent one atom of oxygen
from an oxidising agent and the symbol [H] to represent one atom of hydrogen from a reducing agent)understand and use the following terminology associated with types of organic mechanisms:
(a) free-radical substitution
(b) electrophilic addition
(c) nucleophilic substitution
(d) nucleophilic addition(in organic reaction mechanisms, the use of curly arrows to represent movement of electron pairs is
expected; the arrow should begin at a bond or a lone pair of electrons)
Organic Chemistry Terminology
(a) Homologous Series
A homologous series is a family of organic compounds that have the same functional group, the same general formula, and similar chemical properties, in which successive members differ by a \( \mathrm{CH_2} \) unit.
![]()
Key Points
- All members contain the same functional group
- They share the same general formula
- They undergo similar chemical reactions
- Successive members differ in molecular mass by 14
- They show a gradual change in physical properties
At A level, you must clearly state the presence of a CH₂ difference when identifying a homologous series.
Example
Explain why ethanol and propan-1-ol belong to the same homologous series.
▶️ Answer / Explanation
Both contain the –OH functional group.
They have the same general formula and differ by a \( \mathrm{CH_2} \) unit.
(b) Saturated and Unsaturated
Saturated organic compounds contain only single carbon–carbon bonds and therefore have the maximum possible number of hydrogen atoms.
Unsaturated organic compounds contain one or more carbon–carbon multiple bonds, such as C=C or C≡C, and therefore have fewer hydrogen atoms.
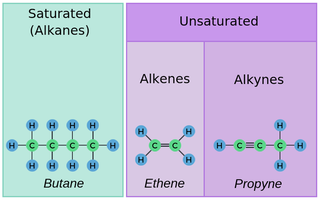
Key Points
- Alkanes are saturated hydrocarbons
- Alkenes and alkynes are unsaturated hydrocarbons
- Unsaturated compounds are generally more reactive
- Unsaturated compounds undergo addition reactions
- Saturated compounds do not undergo addition reactions easily
At A level, the presence of a C=C or C≡C bond must be explicitly stated to describe a compound as unsaturated.
Example
Explain why hexene is described as an unsaturated compound.
▶️ Answer / Explanation
Hexene contains a carbon–carbon double bond.
This means it is unsaturated.
(c) Homolytic and Heterolytic Fission
Homolytic fission is the breaking of a covalent bond in which the shared pair of electrons is split equally, so that each atom receives one electron. This produces two free radicals.
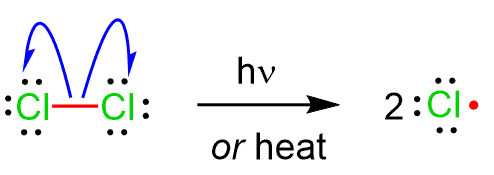
Heterolytic fission is the breaking of a covalent bond in which the shared pair of electrons is split unequally, so that both electrons go to one atom. This produces a positive ion (cation) and a negative ion (anion).

Key Points
- Homolytic fission produces free radicals
- It usually occurs in non-polar bonds
- Energy is required, often supplied by UV light or heat
- Heterolytic fission produces ions
- It usually occurs in polar bonds
- The more electronegative atom takes both electrons in heterolytic fission
Link to Reactions
- Homolytic fission is involved in free-radical substitution reactions
- Heterolytic fission is involved in nucleophilic and electrophilic reactions
At A level, you must clearly state how the bonding electrons are split when describing bond fission.
Example
Explain the difference between homolytic and heterolytic fission of a C–Br bond.
▶️ Answer / Explanation
In homolytic fission, each atom receives one electron, forming two radicals.
In heterolytic fission, both electrons go to the bromine atom, forming ions.
(d) Free Radical, Initiation, Propagation, Termination
A free radical is a highly reactive species that contains an unpaired electron. Free radicals are represented using a dot to show the unpaired electron.

Free Radical Reactions
Initiation is the step in which free radicals are first formed, usually by homolytic fission caused by ultraviolet light or heat.
Propagation steps involve free radicals reacting with molecules to form new radicals, allowing the reaction to continue as a chain reaction.
Termination steps occur when two free radicals react together, removing radicals and stopping the chain reaction.
Key Points
- Free radicals are neutral but very reactive
- They are short-lived due to the unpaired electron
- Propagation involves both breaking and forming bonds
- Termination removes radicals permanently
- Free radical reactions often give mixtures of products
At A level, you must be able to identify initiation, propagation, and termination steps in a reaction mechanism.
Example
In the free-radical substitution of methane with chlorine, identify one termination step.
▶️ Answer / Explanation
A termination step is the combination of two radicals.
For example, a methyl radical reacting with a chlorine radical to form chloromethane.
(e) Nucleophile, Electrophile, Nucleophilic and Electrophilic
A nucleophile is a species that donates a lone pair of electrons to form a new covalent bond. 
An electrophile is a species that accepts a lone pair of electrons to form a new covalent bond.
A nucleophilic reaction is one in which a nucleophile attacks an electron-deficient centre.
An electrophilic reaction is one in which an electrophile attacks an electron-rich centre.
Key Points
- Nucleophiles are usually negatively charged or have lone pairs
- Electrophiles are often positively charged or electron-deficient
- Nucleophiles attack areas of partial positive charge
- Electrophiles attack areas of high electron density
- The terms describe how species behave in reactions
Common Examples
- \( \mathrm{OH^-} \), \( \mathrm{CN^-} \), \( \mathrm{NH_3} \) are nucleophiles
- \( \mathrm{H^+} \), \( \mathrm{NO_2^+} \), electron-deficient carbon atoms act as electrophiles
At A level, you must identify nucleophiles and electrophiles based on electron movement, not just charge.
Example
Explain why the hydroxide ion, \( \mathrm{OH^-} \), acts as a nucleophile in organic reactions.
▶️ Answer / Explanation
The hydroxide ion has a lone pair of electrons.
It can donate this electron pair to form a covalent bond.
(f) Types of Organic Reactions
Organic reactions can be classified according to the overall change that occurs in the reactants. Correctly identifying the reaction type is essential at A level.
Addition
An addition reaction is one in which two molecules combine to form a single product, usually involving an unsaturated compound.
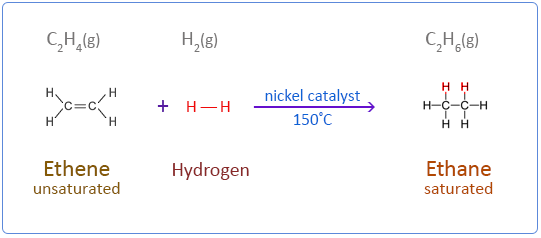
- Occurs at C=C or C≡C bonds
- No atoms are lost from the reactants
- Common in alkenes and alkynes
Substitution
A substitution reaction is one in which one atom or group is replaced by another.

- The carbon skeleton remains unchanged
- Typical of saturated compounds
- Includes nucleophilic and free-radical substitution
Elimination
An elimination reaction is one in which a small molecule is removed from a larger molecule to form a multiple bond.
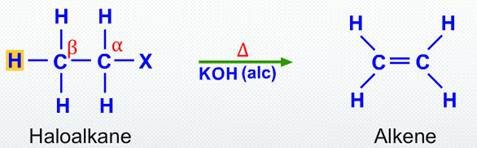
- Often the reverse of addition
- Produces alkenes from saturated compounds
- Commonly involves loss of water or hydrogen halide
Hydrolysis
Hydrolysis is a reaction in which a bond is broken using water or aqueous conditions.
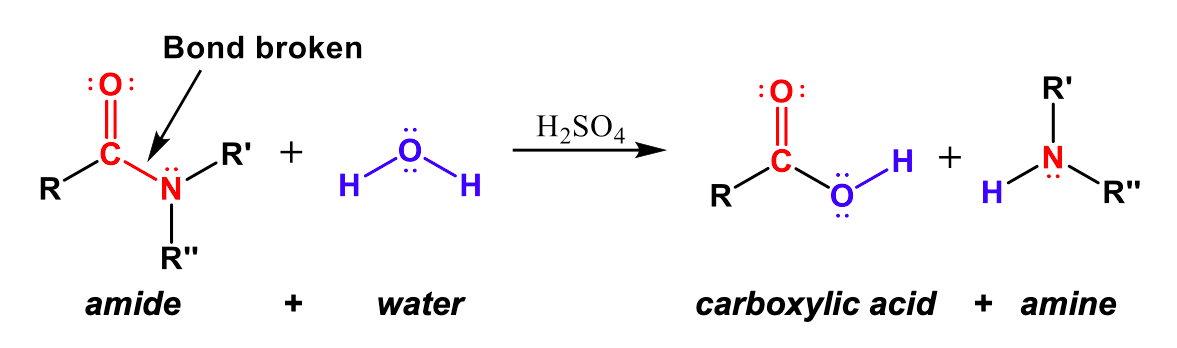
- Water is a reactant
- Common in esters, halogenoalkanes, and amides
- Often carried out using acid or alkali
Condensation
A condensation reaction is one in which two molecules combine with the loss of a small molecule, usually water.
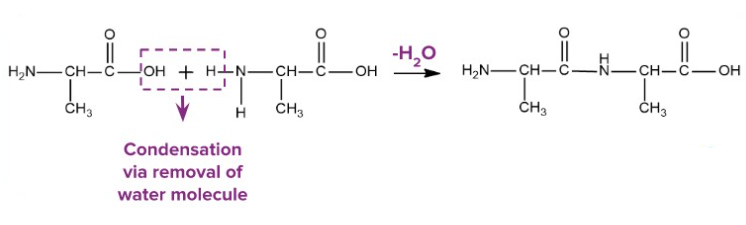
- Opposite of hydrolysis
- Forms larger, more complex molecules
- Important in ester and polymer formation
At A level, always identify the reaction type by comparing the number of reactants and products and checking whether atoms are replaced, added, or removed.
Example
When ethanol is heated with concentrated sulfuric acid, ethene and water are formed. Identify the type of reaction and justify your answer.
▶️ Answer / Explanation
This is an elimination reaction.
Water is removed from ethanol, forming a C=C double bond.
(g) Oxidation and Reduction (Organic Chemistry)
In organic chemistry, oxidation and reduction are defined in terms of oxygen and hydrogen transfer rather than electron transfer.
Oxidation is the gain of oxygen or the loss of hydrogen.
![]()
Reduction is the loss of oxygen or the gain of hydrogen.
![]()
Key Points
- Organic redox reactions do not involve free electrons
- Oxidation and reduction always occur together
- The oxidation state of carbon increases during oxidation
- The oxidation state of carbon decreases during reduction
- Common oxidising agents include acidified potassium dichromate
Use of [O] and [H]
In organic equations, the symbol [O] represents one oxygen atom supplied by an oxidising agent.
The symbol [H] represents one hydrogen atom supplied by a reducing agent.
These symbols simplify equations where the oxidising or reducing agent is not shown explicitly.
Example
Use [O] to show the oxidation of ethanol to ethanoic acid.
▶️ Answer / Explanation
Ethanol + 2[O] → ethanoic acid + water
Ethanol gains oxygen, so it is oxidised.
(a) Free-Radical Substitution
Free-radical substitution is a reaction mechanism in which a hydrogen atom in an alkane is replaced by another atom or group, via the formation and reaction of free radicals.
Key Features
- Occurs mainly in alkanes
- Involves homolytic fission
- Requires UV light or heat to initiate the reaction
- Proceeds via a chain reaction
- Produces a mixture of products
Mechanism Stages
Initiation – homolytic fission of a halogen molecule to form free radicals.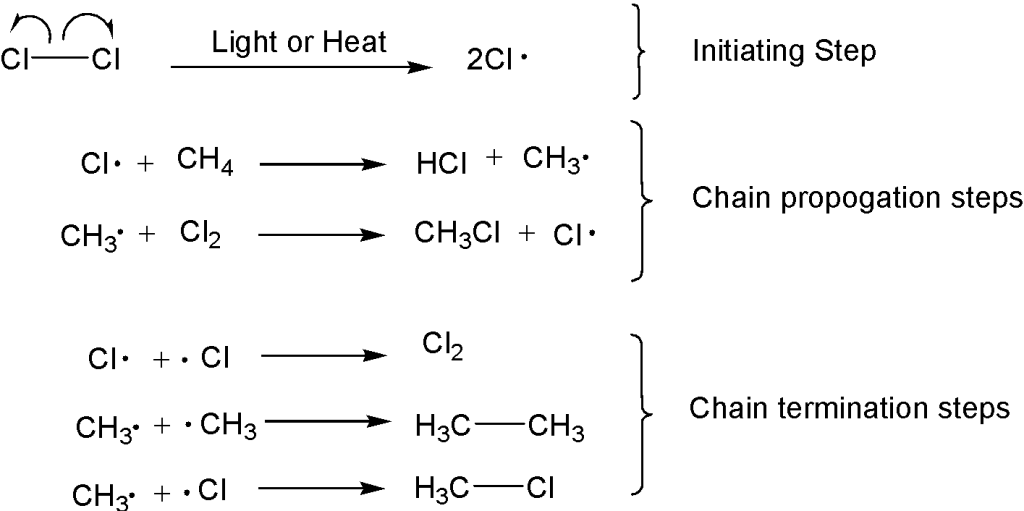
\( \mathrm{Cl_2 \rightarrow 2Cl\cdot} \)
Propagation – free radicals react to form new radicals and products.
\( \mathrm{CH_4 + Cl\cdot \rightarrow CH_3\cdot + HCl} \)
\( \mathrm{CH_3\cdot + Cl_2 \rightarrow CH_3Cl + Cl\cdot} \)
Termination – two radicals combine to form a stable molecule.
\( \mathrm{CH_3\cdot + Cl\cdot \rightarrow CH_3Cl} \)
Curly Arrows
In free-radical mechanisms, single-headed (fish-hook) arrows are used to show the movement of single electrons.
At A level, curly arrows must start from a bond to show homolytic fission, or from a radical to show movement of an unpaired electron.
Example
Explain why UV light is required for the chlorination of methane.
▶️ Answer / Explanation
UV light provides the energy needed to break the Cl–Cl bond by homolytic fission.
This produces chlorine radicals that initiate the reaction.
(b) Electrophilic Addition
Electrophilic addition is a reaction mechanism in which an electrophile attacks an electron-rich region of a molecule, typically a C=C double bond, resulting in the addition of atoms across the double bond.
Key Features
- Occurs mainly in alkenes
- The C=C bond acts as a region of high electron density
- Involves an electrophile in the first step
- The double bond breaks and two new single bonds form
- Converts an unsaturated compound into a saturated one
Mechanism Outline
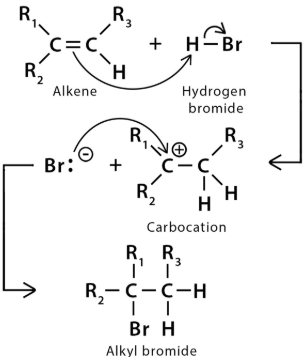
Step 1: The electrophile is attracted to the C=C bond and reacts with it.
Step 2: The double bond breaks and a new bond forms between the electrophile and one carbon atom.
Curly Arrows
In electrophilic addition mechanisms, full curly arrows are used to show the movement of an electron pair.
The arrow must start at the π bond of the C=C and point towards the electrophile.
Link to Reactions
- Addition of hydrogen halides to alkenes
- Addition of halogens (e.g. bromine)
- Addition of sulfuric acid to alkenes
At A level, you must show correct use of curly arrows starting from electron-rich regions.
Example
Explain why ethene undergoes electrophilic addition with bromine.
▶️ Answer / Explanation
The C=C double bond in ethene is electron-rich.
It attracts the electrophile and allows bromine to add across the double bond.
(c) Nucleophilic Substitution
Nucleophilic substitution is a reaction mechanism in which a nucleophile attacks an electron-deficient carbon atom and replaces another atom or group, usually a halogen.
Key Features
- Occurs mainly in halogenoalkanes
- Involves a nucleophile with a lone pair of electrons
- The C–X bond is polar, making carbon electron-deficient
- The halogen leaves as a halide ion
- The carbon skeleton remains unchanged
Mechanism Outline
Step 1: The nucleophile is attracted to the partially positive carbon atom.
Step 2: The nucleophile donates a lone pair to form a new bond while the C–X bond breaks.
Curly Arrows
In nucleophilic substitution, full curly arrows are used to show the movement of an electron pair.
One arrow must start from the lone pair on the nucleophile, and another from the C–X bond towards the halogen.
Link to Reactions
- Reaction of halogenoalkanes with \( \mathrm{OH^-} \)
- Reaction of halogenoalkanes with \( \mathrm{NH_3} \)
- Hydrolysis of halogenoalkanes
At A level, curly arrows must clearly show where the electron pair comes from and where it goes.
Example
Explain why 1-bromopropane undergoes nucleophilic substitution with \( \mathrm{OH^-} \).
▶️ Answer / Explanation
The C–Br bond is polar, making the carbon atom partially positive.
The hydroxide ion donates a lone pair and replaces the bromine atom.
(d) Nucleophilic Addition
Nucleophilic addition is a reaction mechanism in which a nucleophile attacks an electron-deficient carbon atom in a polar multiple bond, resulting in the addition of atoms across the double bond.
Key Features
- Occurs mainly in aldehydes and ketones

- Involves a polar C=O bond
- The carbon atom is electron-deficient
- The carbon–oxygen double bond is broken
- No atoms are removed from the molecule
Mechanism Outline
Step 1: The nucleophile is attracted to the partially positive carbon atom of the C=O bond.
Step 2: A new bond forms between the nucleophile and carbon, and the π bond breaks.
Curly Arrows
Full curly arrows are used to show the movement of electron pairs.
One arrow starts from the lone pair on the nucleophile, and another from the C=O π bond towards the oxygen atom.
Link to Reactions
- Addition of cyanide ions to aldehydes and ketones
- Reduction of carbonyl compounds by hydride ions
- Reactions involving nucleophilic attack on C=O
At A level, you must clearly show attack by a nucleophile and correct movement of electron pairs using curly arrows.
Example
Explain why the carbon atom in a carbonyl group is susceptible to nucleophilic addition.
▶️ Answer / Explanation
The C=O bond is polar.
The carbon atom has a partial positive charge and attracts nucleophiles.

