CIE AS/A Level Chemistry 13.3 Shapes of organic molecules; $\sigma$ and $\pi$ bonds Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 13.3 Shapes of organic molecules; $\sigma$ and $\pi$ bonds Study Notes – New Syllabus
CIE AS/A Level Chemistry 13.3 Shapes of organic molecules; $\sigma$ and $\pi$ bonds Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe organic molecules as either straight-chained, branched or cyclic
describe and explain the shape of, and bond angles in, molecules containing sp, sp² and sp³ hybridised atoms
describe the arrangement of σ and π bonds in molecules containing sp, sp² and sp³ hybridised atoms
understand and use the term planar when describing the arrangement of atoms in organic molecules, for example ethene
Straight-Chained, Branched and Cyclic Organic Molecules
Organic molecules can be classified according to the shape of their carbon skeleton. At A level, you must be able to describe molecules as straight-chained, branched, or cyclic based on their structure.
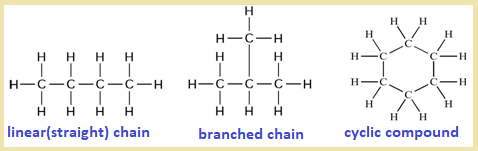
Straight-Chained Molecules
A straight-chained organic molecule is one in which the carbon atoms are joined together in a single continuous chain, with no side branches.
- Carbon atoms form one unbroken chain
- No carbon atoms branch off the main chain
- May be saturated or unsaturated
- Often the simplest member of a given molecular formula
Branched Molecules
A branched organic molecule is one in which one or more carbon atoms branch off from the main carbon chain.
- Contain a main carbon chain and one or more side chains
- Still belong to the same homologous series as straight-chain isomers
- Have the same molecular formula as straight-chain isomers
- Often have lower boiling points than straight-chain isomers
Cyclic Molecules
A cyclic organic molecule is one in which the carbon atoms are joined together to form a ring structure.
- The carbon chain forms a closed loop
- May be saturated or unsaturated
- Do not have ends to the carbon chain
- Include cycloalkanes and aromatic compounds
At A level, classification depends on the carbon skeleton, not the functional group.
Example
State whether hexane is straight-chained, branched, or cyclic.
▶️ Answer / Explanation
Hexane is a straight-chained molecule.
All six carbon atoms form a single continuous chain.
Example
Explain why 2-methylpropane is described as a branched molecule.
▶️ Answer / Explanation
The molecule has a main carbon chain with a methyl group attached as a side branch.
This creates a branched carbon skeleton.
Example
Compound X has the molecular formula \( \mathrm{C_6H_{12}} \) and forms a ring structure. Classify the molecule and justify your answer.
▶️ Answer / Explanation
The compound is cyclic.
The carbon atoms are joined in a closed ring rather than a straight or branched chain.
Shapes and Bond Angles in Hybridised Molecules
The shape and bond angles of organic molecules depend on the type of hybridisation of the carbon atom. At A level, you must be able to describe and explain molecular shape using sp, sp², and sp³ hybridisation.
sp Hybridisation
A carbon atom is sp hybridised when it forms two sigma (\( \sigma \)) bonds and two pi (\( \pi \)) bonds.
![]()
- Hybridisation: one s orbital + one p orbital
- Number of bonding regions: 2
- Molecular shape: linear
- Bond angle: 180°
- Found in alkynes and nitriles
The linear shape results from two regions of electron density repelling each other equally.
sp² Hybridisation
A carbon atom is sp² hybridised when it forms three sigma (\( \sigma \)) bonds and one pi (\( \pi \)) bond.
![]()
- Hybridisation: one s orbital + two p orbitals
- Number of bonding regions: 3
- Molecular shape: trigonal planar
- Bond angle: 120°
- Found in alkenes and carbonyl groups
The planar arrangement minimises repulsion between three regions of electron density.
sp³ Hybridisation
A carbon atom is sp³ hybridised when it forms four sigma (\( \sigma \)) bonds and has no lone pairs.
![]()
- Hybridisation: one s orbital + three p orbitals
- Number of bonding regions: 4
- Molecular shape: tetrahedral
- Bond angle: 109.5°
- Found in alkanes
The tetrahedral shape occurs because electron pairs repel each other and arrange themselves as far apart as possible.
At A level, you should always link hybridisation → number of bonding regions → shape → bond angle.
Summary Table
| Hybridisation | Number of bonding regions | Orbitals mixed | Molecular shape | Bond angle | Typical example |
|---|---|---|---|---|---|
| sp³ | 4 | 1 s + 3 p | Tetrahedral | 109.5° | Methane |
| sp² | 3 | 1 s + 2 p | Trigonal planar | 120° | Ethene |
| sp | 2 | 1 s + 1 p | Linear | 180° | Ethyne |
Example
State the shape and bond angle around the carbon atom in methane.
▶️ Answer / Explanation
The carbon atom is sp³ hybridised.
The shape is tetrahedral with a bond angle of 109.5°.
Example
Describe the shape around each carbon atom in ethene.
▶️ Answer / Explanation
Each carbon atom is sp² hybridised.
The shape is trigonal planar with bond angles of 120°.
Example
Explain why the bond angle around the carbon atom in ethyne is greater than in ethene.
▶️ Answer / Explanation
In ethyne, carbon is sp hybridised with two bonding regions.
This gives a linear shape with a bond angle of 180°, which is larger than the 120° bond angle in ethene.
Arrangement of \( \sigma \) and \( \pi \) Bonds in Hybridised Molecules
In organic molecules, covalent bonds can be classified as sigma (\( \sigma \)) bonds or pi (\( \pi \)) bonds. The type and arrangement of these bonds depend on whether atoms are sp, sp², or sp³ hybridised.
Sigma (\( \sigma \)) Bonds
A sigma bond is formed by the end-on overlap of atomic or hybrid orbitals along the line joining the two nuclei.
![]()
- All single bonds are sigma bonds
- Sigma bonds allow free rotation about the bond axis
- Sigma bonds are stronger than pi bonds
Pi (\( \pi \)) Bonds
A pi bond is formed by the sideways overlap of unhybridised p orbitals above and below the plane of the molecule.
![]()
- Present only in double and triple bonds
- Restrict rotation about the bond
- Weaker than sigma bonds
sp Hybridised Atoms
In sp hybridisation, one s orbital and one p orbital combine to form two sp hybrid orbitals, leaving two unhybridised p orbitals.
- Forms two sigma (\( \sigma \)) bonds
- Forms two pi (\( \pi \)) bonds
- The two pi bonds are perpendicular to each other
- Example: alkynes and nitriles
A carbon–carbon triple bond consists of one \( \sigma \) bond and two \( \pi \) bonds.
sp² Hybridised Atoms
In sp² hybridisation, one s orbital and two p orbitals combine to form three sp² hybrid orbitals, leaving one unhybridised p orbital.
- Forms three sigma (\( \sigma \)) bonds
- Forms one pi (\( \pi \)) bond
- The \( \pi \) bond lies above and below the plane of the molecule
- Example: alkenes and carbonyl groups
A carbon–carbon double bond consists of one \( \sigma \) bond and one \( \pi \) bond.
sp³ Hybridised Atoms
In sp³ hybridisation, one s orbital and three p orbitals combine to form four sp³ hybrid orbitals.
- Forms four sigma (\( \sigma \)) bonds
- No pi bonds are present
- All bonds are single bonds
- Example: alkanes
Each sp³ orbital overlaps end-on with another orbital to form a sigma bond.
Summary Table
| Hybridisation | \( \sigma \) bonds | \( \pi \) bonds | Bond type present | Typical example |
|---|---|---|---|---|
| sp³ | 4 | 0 | Single bonds only | Methane |
| sp² | 3 | 1 | One double bond | Ethene |
| sp | 2 | 2 | One triple bond | Ethyne |
Example
State the number of \( \sigma \) and \( \pi \) bonds around each carbon atom in ethane.
▶️ Answer / Explanation
Each carbon atom forms four \( \sigma \) bonds.
No \( \pi \) bonds are present.
Example
Describe the arrangement of \( \sigma \) and \( \pi \) bonds in a carbon–carbon double bond.
▶️ Answer / Explanation
A C=C double bond contains one \( \sigma \) bond and one \( \pi \) bond.
The \( \pi \) bond is formed by sideways overlap of p orbitals.
Example
Explain why rotation is restricted about a carbon–carbon double bond.
▶️ Answer / Explanation
Rotation would break the \( \pi \) bond formed by sideways overlap of p orbitals.
Breaking the \( \pi \) bond requires energy, so rotation is restricted.
Planar Arrangement in Organic Molecules
The term planar is used in organic chemistry to describe a molecule in which all the atoms lie in the same flat plane. At A level, you must understand and correctly use this term when describing the structure of molecules such as ethene.
Definition
A molecule is described as planar when all its atoms are arranged in a single plane, with no atoms above or below that plane.
Planarity and Hybridisation
- Planar molecules usually contain sp² hybridised atoms
- sp² hybridisation gives a trigonal planar arrangement
- Bond angles are approximately 120°
- The presence of a \( \pi \) bond requires orbitals to overlap sideways
- This sideways overlap is only possible when atoms lie in the same plane
Example: Ethene
In ethene, each carbon atom is sp² hybridised.
- Each carbon forms three \( \sigma \) bonds
- The remaining p orbitals overlap sideways to form a \( \pi \) bond
- All six atoms lie in the same plane
- The molecule is therefore planar
At A level, always link planar shape → sp² hybridisation → presence of a \( \pi \) bond.
Example
What does the term planar mean when describing an organic molecule?
▶️ Answer / Explanation
All the atoms lie in the same flat plane.
Example
Explain why ethene is described as a planar molecule.
▶️ Answer / Explanation
Each carbon atom is sp² hybridised.
This gives a trigonal planar arrangement with all atoms in the same plane.
Example
Explain why rotation is restricted in ethene and relate this to its planar structure.
▶️ Answer / Explanation
Ethene contains a \( \pi \) bond formed by sideways overlap of p orbitals.
Rotation would break the \( \pi \) bond, so the atoms remain fixed in a planar arrangement.
