CIE AS/A Level Chemistry 13.4 Isomerism: structural and stereoisomerism Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 13.4 Isomerism: structural and stereoisomerism Study Notes – New Syllabus
CIE AS/A Level Chemistry 13.4 Isomerism: structural and stereoisomerism Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe structural isomerism and its division into chain, positional and functional group isomerism
describe stereoisomerism and its division into geometrical (cis/trans) and optical isomerism (use of E/Z nomenclature is acceptable but is not required)
describe geometrical (cis/trans) isomerism in alkenes, and explain its origin in terms of restricted rotation due to the presence of π bonds
explain what is meant by a chiral centre and that such a centre gives rise to two optical isomers (enantiomers)
(Candidates should appreciate that compounds can contain more than one chiral centre, but knowledge of meso compounds, or nomenclature such as diastereoisomers is not required.)
identify chiral centres and geometrical (cis/trans) isomerism in a molecule of given structural formula including cyclic compounds
deduce the possible isomers for an organic molecule of known molecular formula
Structural Isomerism
Structural isomerism occurs when compounds have the same molecular formula but different structural formulae. This means the atoms are connected in different ways.
Structural isomerism can be divided into chain isomerism, positional isomerism, and functional group isomerism.

Chain Isomerism
Chain isomerism occurs when compounds have the same molecular formula but different carbon skeletons due to branching.
- Difference is in the arrangement of the carbon chain
- Functional group remains the same
- More common in larger molecules
- Branched isomers often have lower boiling points
Positional Isomerism
Positional isomerism occurs when compounds have the same carbon skeleton and same functional group, but the functional group is attached at different positions on the carbon chain.
- Carbon chain remains unchanged
- Position of functional group or multiple bond changes
- Leads to different physical and chemical properties
Functional Group Isomerism
Functional group isomerism occurs when compounds have the same molecular formula but different functional groups.
- Compounds belong to different homologous series
- Functional groups are different
- Chemical properties are very different
Example
Define structural isomerism.
▶️ Answer / Explanation
Structural isomerism occurs when compounds have the same molecular formula but different structural formulae.
Example
Explain why butane and 2-methylpropane are chain isomers.
▶️ Answer / Explanation
They have the same molecular formula \( \mathrm{C_4H_{10}} \).
The carbon skeletons are different due to branching.
Example
Propan-1-ol and methoxyethane have the same molecular formula. Identify the type of structural isomerism shown and justify your answer.
▶️ Answer / Explanation
This is functional group isomerism.
Propan-1-ol is an alcohol, while methoxyethane is an ether.
Stereoisomerism
Stereoisomerism occurs when compounds have the same structural formula but differ in the spatial arrangement of atoms in three dimensions.
The bonding between atoms is the same, but the molecules are arranged differently in space. Stereoisomerism is divided into geometrical (cis/trans) isomerism and optical isomerism.
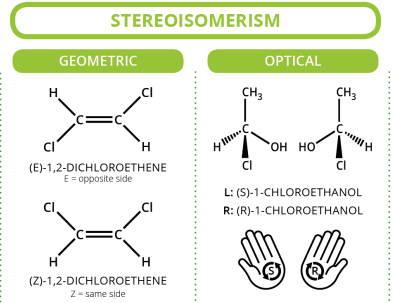
Geometrical (cis/trans) Isomerism
Geometrical isomerism occurs due to restricted rotation about a C=C double bond or within a ring structure.
- Occurs in alkenes and cyclic compounds
- Requires restricted rotation
- Each carbon of the C=C must be bonded to two different groups
- Isomers differ in the relative positions of groups
In cis–trans isomerism:
- cis – similar groups are on the same side of the double bond
- trans – similar groups are on opposite sides of the double bond
The E/Z system may also be used, where priorities are assigned to groups, but cis/trans is sufficient at this level.
Optical Isomerism
Optical isomerism occurs when molecules are non-superimposable mirror images of each other.
These molecules contain a chiral carbon atom, which is a carbon bonded to four different groups.
- Optical isomers are called enantiomers
- They rotate plane-polarised light
- One rotates light clockwise, the other anticlockwise
- They have identical physical properties except optical activity
Example
Define stereoisomerism.
▶️ Answer / Explanation
Stereoisomerism occurs when compounds have the same structural formula but differ in the spatial arrangement of atoms.
Example
Explain why but-2-ene shows geometrical isomerism.
▶️ Answer / Explanation
There is restricted rotation about the C=C bond.
Each carbon atom in the double bond is bonded to two different groups.
Example
Explain why a molecule with a carbon atom bonded to four different groups can show optical isomerism.
▶️ Answer / Explanation
Such a carbon atom is chiral.
The molecule has non-superimposable mirror images that rotate plane-polarised light.
Geometrical (cis/trans) Isomerism in Alkenes
Geometrical isomerism (also known as cis/trans isomerism) is a type of stereoisomerism that occurs in alkenes due to restricted rotation about a carbon–carbon double bond.
Definition
Geometrical isomerism occurs when compounds have the same structural formula but differ in the spatial arrangement of groups around a C=C double bond.
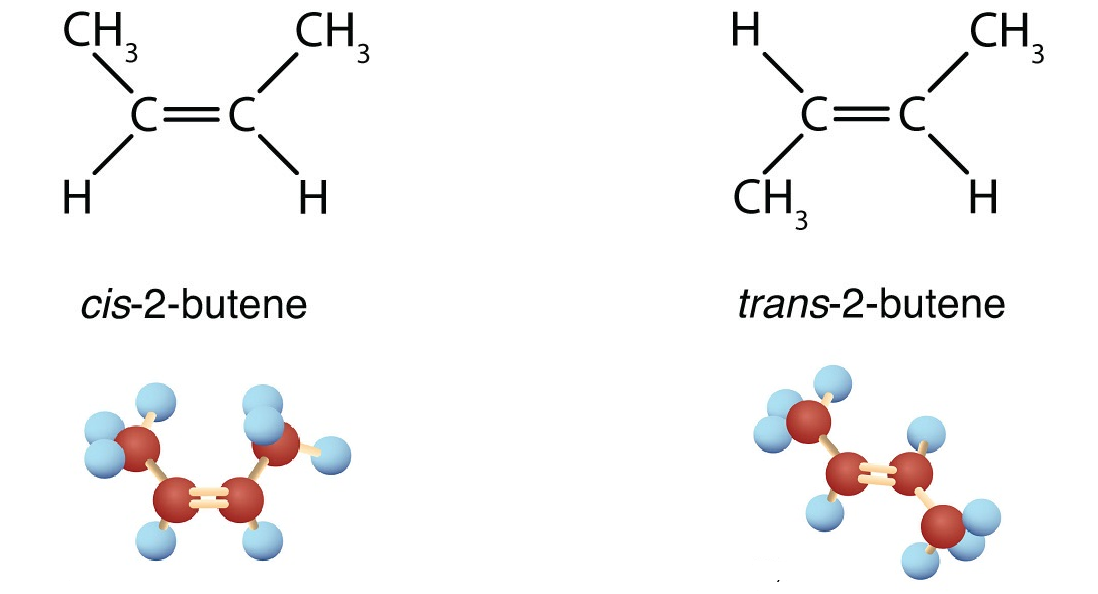
Conditions Required for cis/trans Isomerism
- The molecule must contain a C=C double bond
- Each carbon atom of the double bond must be bonded to two different groups
- Rotation about the double bond must be restricted
If either carbon of the C=C bond has two identical groups attached, cis/trans isomerism is not possible.
cis and trans Forms
In cis isomers, the similar groups are on the same side of the C=C double bond.
In trans isomers, the similar groups are on opposite sides of the C=C double bond.
Origin of Restricted Rotation
A carbon–carbon double bond consists of one sigma (\( \sigma \)) bond and one pi (\( \pi \)) bond.
The \( \pi \) bond is formed by sideways overlap of p orbitals above and below the plane of the molecule.
Rotation around the C=C bond would break the \( \pi \) bond, which requires a large amount of energy.
As a result, rotation is restricted and the relative positions of groups are fixed, leading to geometrical isomerism.
Example
State why rotation about a carbon–carbon double bond is restricted.
▶️ Answer / Explanation
Rotation would break the \( \pi \) bond.
Breaking the \( \pi \) bond requires a lot of energy.
Example
Explain why but-2-ene exists as cis and trans isomers.
▶️ Answer / Explanation
But-2-ene contains a C=C double bond.
Each carbon of the double bond is attached to two different groups.
Rotation is restricted due to the presence of a \( \pi \) bond.
Example
Explain why propene does not show cis/trans isomerism.
▶️ Answer / Explanation
Propene has a C=C double bond.
However, one carbon atom is bonded to two identical hydrogen atoms.
Therefore the conditions for cis/trans isomerism are not met.
Chiral Centre and Optical Isomerism
A chiral centre is a key feature responsible for optical isomerism in organic molecules. At A level, you must be able to identify a chiral centre and explain how it gives rise to two optical isomers (enantiomers).
Chiral Centre
A chiral centre is a carbon atom bonded to four different groups.
This causes the molecule to have no plane of symmetry and results in two possible three-dimensional arrangements.
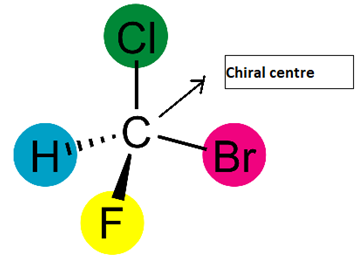
- Usually an sp³ hybridised carbon
- Often marked with an asterisk (*) in structural diagrams
- Not all molecules with four carbon atoms are chiral
Optical Isomers (Enantiomers)
Optical isomers, also called enantiomers, are non-superimposable mirror images of each other.
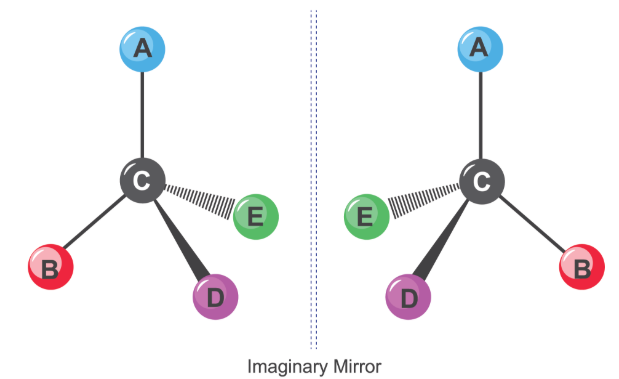
They arise because a chiral centre can be arranged in two distinct ways in three-dimensional space.
- Enantiomers have the same structural and molecular formulae
- They have identical physical properties except optical activity
- Each enantiomer rotates plane-polarised light in opposite directions
At A level, it is sufficient to state that a chiral centre produces two enantiomers.
Why a Chiral Centre Produces Two Enantiomers
Because the carbon atom is bonded to four different groups, there are two possible non-superimposable mirror-image arrangements.
These two arrangements cannot be rotated to overlap exactly, giving rise to two optical isomers.
Example
Define a chiral centre.
▶️ Answer / Explanation
A carbon atom bonded to four different groups.
Example
Explain why a molecule containing a chiral centre can exist as optical isomers.
▶️ Answer / Explanation
The chiral carbon is bonded to four different groups.
This allows two non-superimposable mirror-image arrangements.
Example
Explain why 2-bromobutane shows optical isomerism.
▶️ Answer / Explanation
The second carbon atom is bonded to four different groups.
This carbon atom is a chiral centre, producing two enantiomers.
Identifying Chiral Centres and Geometrical (cis/trans) Isomerism
Identifying a Chiral Centre
A chiral centre is a carbon atom bonded to four different groups.

How to Identify a Chiral Centre
- Look for an sp³ hybridised carbon
- Check all four bonds from that carbon
- Each attached group must be different
- If any two groups are the same, the carbon is not chiral
In cyclic molecules, the two carbon chains on either side of the chiral carbon must be checked carefully — they may look similar but can still be different.
Identifying Geometrical (cis/trans) Isomerism in Alkenes
Geometrical isomerism in alkenes occurs due to restricted rotation about a C=C double bond.

Conditions to Check
- The molecule must contain a C=C double bond
- Each carbon of the double bond must have two different groups attached
- If either carbon has two identical groups, cis/trans isomerism is not possible
Identifying cis/trans Isomerism in Cyclic Compounds
Cyclic compounds can also show cis/trans isomerism because rotation around the ring is restricted.

- Substituents can be on the same side of the ring (cis)
- Or on opposite sides of the ring (trans)
- No C=C bond is required in cyclic cis/trans isomerism
Example
Does the carbon atom in \( \mathrm{CH_3CH(OH)CH_3} \) act as a chiral centre?
▶️ Answer / Explanation
No.
The central carbon is bonded to two identical methyl groups.
Example
Identify whether but-2-ene shows geometrical isomerism.
▶️ Answer / Explanation
Yes.
The molecule contains a C=C double bond.
Each carbon of the double bond is attached to two different groups.
Example
A molecule contains a cyclohexane ring with two methyl groups attached to different carbon atoms. Explain how cis/trans isomerism may arise.
▶️ Answer / Explanation
Rotation within the ring is restricted.
If the methyl groups are on the same side of the ring, the isomer is cis.
If they are on opposite sides, the isomer is trans.
Deducing Possible Isomers from a Molecular Formula

Step 1: Use the Molecular Formula
The molecular formula tells you:
- The total number of each type of atom present
- Possible homologous series (e.g. alkane, alcohol, alkene)
- Whether unsaturation may be present
For example, a formula that fits \( \mathrm{C_{n}H_{2n+2}} \) suggests an alkane, while fewer hydrogens suggest double bonds or rings.
Step 2: Consider Types of Isomerism
When deducing isomers, consider all relevant types:
- Chain isomerism – different carbon skeletons
- Positional isomerism – functional group in different positions
- Functional group isomerism – different functional groups
- Stereoisomerism – cis/trans or optical (if applicable)
Not all types apply to every molecular formula.
Step 3: Draw All Distinct Structures
To ensure all isomers are found:
- Start with the longest possible carbon chain
- Introduce branching where possible
- Move functional groups to all valid positions
- Check that no two structures are identical
Each structure must satisfy correct valency and bonding.
Example
State the number of structural isomers of \( \mathrm{C_4H_{10}} \).
▶️ Answer / Explanation
There are two isomers.
Butane and 2-methylpropane.
Example
Deduce all possible alcohol isomers with the molecular formula \( \mathrm{C_3H_8O} \).
▶️ Answer / Explanation
Two alcohol isomers are possible.
Propan-1-ol and propan-2-ol.
Example
The molecular formula \( \mathrm{C_4H_8O} \) represents several organic compounds. Deduce two different functional group isomers.
▶️ Answer / Explanation
One isomer can be an aldehyde (butanal).
Another can be a ketone (butanone).
They have the same molecular formula but different functional groups.
