CIE AS/A Level Chemistry 14.1 Alkanes Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 14.1 Alkanes Study Notes – New Syllabus
CIE AS/A Level Chemistry 14.1 Alkanes Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which alkanes can be produced:
(a) addition of hydrogen to an alkene in a hydrogenation reaction, H₂(g) and Pt/Ni catalyst and heat
(b) cracking of a longer chain alkane, heat with Al₂O₃describe:
(a) the complete and incomplete combustion of alkanes
(b) the free-radical substitution of alkanes by Cl₂ or Br₂ in the presence of ultraviolet light, as exemplified by the reactions of ethanedescribe the mechanism of free-radical substitution with reference to the initiation, propagation and termination steps
suggest how cracking can be used to obtain more useful alkanes and alkenes of lower Mᵣ from heavier crude oil fractions
understand the general unreactivity of alkanes, including towards polar reagents in terms of the strength of the C–H bonds and their relative lack of polarity
recognise the environmental consequences of carbon monoxide, oxides of nitrogen and unburnt hydrocarbons arising from the combustion of alkanes in the internal combustion engine and of their catalytic removal
Preparation of Alkanes
Alkanes can be prepared by specific reactions that either remove unsaturation or break larger molecules into smaller ones. At A level, you must be able to recall the reagents and conditions for each method.
(a) Hydrogenation of Alkenes
Hydrogenation is an addition reaction in which hydrogen is added across a C=C double bond to form an alkane.

Reagents and Conditions
- Hydrogen gas, \( \mathrm{H_2(g)} \)
- Nickel (Ni) or platinum (Pt) catalyst
- Heat (approximately 150–200 °C)
The alkene is converted into an alkane by breaking the \( \pi \) bond and forming two new \( \sigma \) bonds.
Example equation:
\( \mathrm{C_2H_4 + H_2 \rightarrow C_2H_6} \)
(b) Cracking of Longer-Chain Alkanes
Cracking is a process in which long-chain alkanes are broken down into shorter alkanes and alkenes.
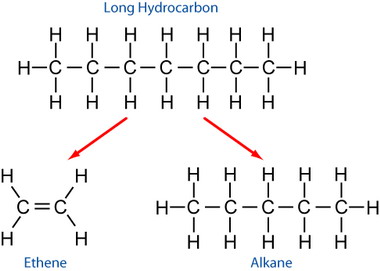
Reagents and Conditions
- High temperature (heat)
- Aluminium oxide catalyst, \( \mathrm{Al_2O_3} \)
- No oxygen present
Cracking produces a mixture of hydrocarbons, including at least one alkane.
Example equation:
\( \mathrm{C_{10}H_{22} \rightarrow C_8H_{18} + C_2H_4} \)
Example
State the reagents needed to convert an alkene into an alkane.
▶️ Answer / Explanation
Hydrogen gas with a nickel or platinum catalyst and heat.
Example
Explain why hydrogenation is described as an addition reaction.
▶️ Answer / Explanation
Hydrogen atoms add across the C=C double bond.
Two reactants combine to form one product.
Example
Explain why cracking requires high temperature and a catalyst.
▶️ Answer / Explanation
High temperature provides energy to break C–C bonds.
The catalyst lowers the activation energy of the reaction.
Reactions of Alkanes
(a) Complete and Incomplete Combustion of Alkanes
Combustion is a reaction in which an alkane reacts with oxygen. Alkanes are used as fuels because combustion releases a large amount of energy.
Complete Combustion
Complete combustion occurs when there is an excess of oxygen.

- All carbon atoms are converted into carbon dioxide
- All hydrogen atoms are converted into water
- Produces the maximum amount of energy
General equation for complete combustion of an alkane:
\( \mathrm{C_nH_{2n+2} + \frac{3n+1}{2}O_2 \rightarrow nCO_2 + (n+1)H_2O} \)
Example (ethane):
\( \mathrm{2C_2H_6 + 7O_2 \rightarrow 4CO_2 + 6H_2O} \)
Incomplete Combustion
Incomplete combustion occurs when there is a limited supply of oxygen.

- Carbon monoxide or carbon (soot) may be produced
- Less energy is released compared to complete combustion
- Carbon monoxide is toxic
Examples (ethane):
\( \mathrm{2C_2H_6 + 5O_2 \rightarrow 4CO + 6H_2O} \)
\( \mathrm{2C_2H_6 + 3O_2 \rightarrow 4C + 6H_2O} \)
(b) Free-Radical Substitution of Alkanes
Free-radical substitution is a reaction in which a hydrogen atom in an alkane is replaced by a halogen atom, such as chlorine or bromine.
This reaction occurs in the presence of ultraviolet (UV) light.
Reagents and Conditions
- Chlorine, \( \mathrm{Cl_2} \), or bromine, \( \mathrm{Br_2} \)
- Ultraviolet light
- Alkane
Example: Reaction of Ethane with Chlorine
Overall reaction:
\( \mathrm{C_2H_6 + Cl_2 \rightarrow C_2H_5Cl + HCl} \)
Mechanism Stages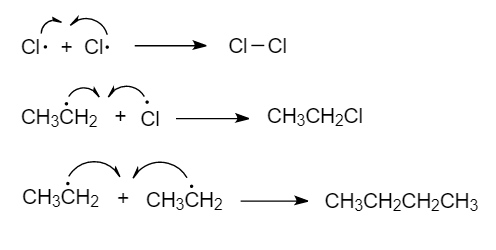
Initiation – homolytic fission of the halogen molecule:
\( \mathrm{Cl_2 \rightarrow 2Cl\cdot} \)
Propagation – chain reaction steps:
\( \mathrm{C_2H_6 + Cl\cdot \rightarrow C_2H_5\cdot + HCl} \)
\( \mathrm{C_2H_5\cdot + Cl_2 \rightarrow C_2H_5Cl + Cl\cdot} \)
Termination – radicals combine:
\( \mathrm{Cl\cdot + Cl\cdot \rightarrow Cl_2} \)
Free-radical substitution produces a mixture of products due to further substitution.
Example
State the products of the complete combustion of an alkane.
▶️ Answer / Explanation
Carbon dioxide and water.
Example
Explain why incomplete combustion of alkanes is dangerous.
▶️ Answer / Explanation
Carbon monoxide is produced.
Carbon monoxide is toxic.
Example
Explain why the reaction of ethane with chlorine must be carried out in UV light.
▶️ Answer / Explanation
UV light provides energy for homolytic fission of the Cl–Cl bond.
This forms chlorine radicals that initiate the reaction.
Mechanism of Free-Radical Substitution
Free-radical substitution is a reaction mechanism in which a hydrogen atom in an alkane is replaced by another atom (usually a halogen) via a chain reaction involving free radicals.
Overview of the Mechanism
- Occurs in the presence of ultraviolet (UV) light
- Involves free radicals (species with an unpaired electron)
- Proceeds via three stages: initiation, propagation, and termination

Initiation
The initiation step is where free radicals are first formed. This occurs by homolytic fission of a halogen molecule when exposed to UV light.
Example:
\( \mathrm{Cl_2 \xrightarrow{UV} 2Cl\cdot} \)
Each chlorine atom takes one electron from the shared pair, forming two chlorine radicals.
Propagation
Propagation steps are the main reactions of the mechanism. In these steps, radicals are used up and regenerated, allowing the chain reaction to continue.
Example using ethane:
\( \mathrm{C_2H_6 + Cl\cdot \rightarrow C_2H_5\cdot + HCl} \)
\( \mathrm{C_2H_5\cdot + Cl_2 \rightarrow C_2H_5Cl + Cl\cdot} \)
- A hydrogen atom is removed from the alkane
- An alkyl radical is formed
- A new halogen radical is regenerated
Termination
Termination steps occur when two free radicals react together, forming a stable molecule and removing radicals from the system.
Examples:
\( \mathrm{Cl\cdot + Cl\cdot \rightarrow Cl_2} \)
\( \mathrm{C_2H_5\cdot + Cl\cdot \rightarrow C_2H_5Cl} \)
\( \mathrm{C_2H_5\cdot + C_2H_5\cdot \rightarrow C_4H_{10}} \)
Termination stops the chain reaction because no radicals remain.
Key Exam Points
- Initiation produces radicals
- Propagation regenerates radicals
- Termination removes radicals
- UV light is required only for the initiation step
- Free-radical substitution produces a mixture of products
Example
Which step of free-radical substitution requires UV light?
▶️ Answer / Explanation
The initiation step.
Example
Explain why propagation steps are described as a chain reaction.
▶️ Answer / Explanation
Radicals are regenerated in propagation steps.
This allows the reaction to continue repeatedly.
Example
Explain why termination steps reduce the rate of free-radical substitution.
▶️ Answer / Explanation
Termination removes free radicals.
With fewer radicals available, fewer propagation steps occur.
Use of Cracking to Obtain Useful Alkanes and Alkenes
Cracking is an industrial process used to convert heavy, less useful hydrocarbons in crude oil fractions into smaller alkanes and alkenes with lower relative molecular masses. These smaller molecules are more useful as fuels and chemical feedstocks.
Why Cracking is Needed
- Heavy fractions contain long-chain alkanes with high boiling points
- These hydrocarbons are viscous and burn inefficiently
- There is greater demand for short-chain fuels
- Alkenes are needed to make polymers and other chemicals
How Cracking Works
Cracking involves heating long-chain alkanes to very high temperatures, causing C–C bonds to break.
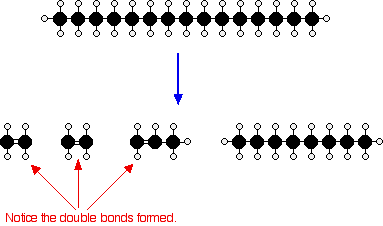
This produces a mixture of shorter-chain alkanes and alkenes.
Typical Conditions
- High temperature (around 500–700 °C)

- Catalyst such as aluminium oxide, \( \mathrm{Al_2O_3} \)
- No oxygen present
Catalysts lower the activation energy, making the process more efficient.
Advantages of the Products
- Short-chain alkanes are useful fuels
- They are more volatile and ignite easily
- Alkenes are valuable feedstocks for chemical synthesis
- Used to make plastics, alcohols, and other compounds
Example Reaction
Cracking of a long-chain alkane:
\( \mathrm{C_{12}H_{26} \rightarrow C_8H_{18} + C_4H_8} \)
At A level, it is sufficient to state that cracking produces a mixture of products.
Example
State why cracking is used in the petroleum industry.
▶️ Answer / Explanation
To convert long-chain hydrocarbons into more useful short-chain alkanes and alkenes.
Example
Explain why short-chain alkanes obtained from cracking are better fuels than long-chain alkanes.
▶️ Answer / Explanation
They are more volatile.
They ignite more easily and burn more cleanly.
Example
A heavy crude oil fraction contains mainly long-chain alkanes. Explain how cracking can make this fraction more useful.
▶️ Answer / Explanation
Cracking breaks long-chain alkanes into smaller alkanes and alkenes.
The products have lower relative molecular masses.
They are more valuable as fuels and chemical feedstocks.
Unreactivity of Alkanes
Alkanes are generally described as unreactive compounds. At A level, you must be able to explain this unreactivity in terms of the strength of C–H and C–C bonds and the lack of polarity in alkane molecules, particularly when considering reactions with polar reagents.
Strong Covalent Bonds in Alkanes
Alkanes contain only C–C and C–H bonds.
- C–C and C–H bonds are strong covalent bonds
- A large amount of energy is required to break these bonds
- As a result, alkanes do not react easily
This is why reactions involving alkanes usually require high temperatures or UV light.
Lack of Polarity in Alkanes
The difference in electronegativity between carbon and hydrogen is very small.
- C–H bonds are almost non-polar
- There are no significant partial charges (\( \delta^+ \) or \( \delta^- \))
- Alkanes do not attract polar reagents
Because alkanes are non-polar, they do not react readily with nucleophiles or electrophiles.
Lack of Reactive Sites
Alkanes contain only single \( \sigma \) bonds and no functional groups.
- No regions of high or low electron density
- No double or triple bonds
- No polar functional groups
This further contributes to their low reactivity.
Summary Explanation
Alkanes are unreactive because they contain strong, non-polar C–H and C–C bonds and have no functional groups to attract reagents.
At A level, answers must mention strong C–H bonds and lack of polarity to gain full marks.
Example
State why alkanes are generally unreactive.
▶️ Answer / Explanation
They contain strong C–H and C–C bonds.
The bonds are non-polar.
Example
Explain why alkanes do not react readily with polar reagents.
▶️ Answer / Explanation
Alkanes are non-polar.
They contain no significant partial charges to attract polar reagents.
Example
Explain why alkanes usually require UV light or high temperatures to react.
▶️ Answer / Explanation
Strong C–H bonds require a large amount of energy to break.
UV light or heat provides the energy needed to start reactions.
Environmental Consequences of Combustion Products from Alkanes
In an internal combustion engine, alkanes are burned as fuels. However, combustion is often incomplete and occurs at high temperatures, producing harmful pollutants. At A level, you must be able to recognise the environmental consequences of these pollutants and understand their catalytic removal.
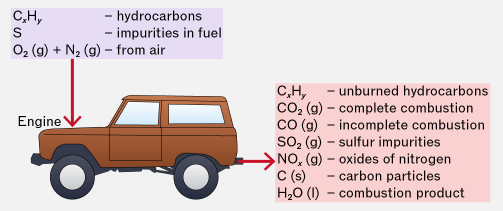
Carbon Monoxide (CO)
Carbon monoxide is produced by the incomplete combustion of alkanes when there is insufficient oxygen.
- Colourless, odourless, and toxic gas
- Binds strongly to haemoglobin in blood
- Reduces oxygen transport in the body
- Can cause headaches, unconsciousness, and death
Carbon monoxide is a major health hazard in urban areas with heavy traffic.
Oxides of Nitrogen (NO and NO₂)
Oxides of nitrogen (collectively called NOx) are formed when nitrogen and oxygen from air react at the high temperatures inside an engine.
- Contribute to the formation of acid rain
- Cause respiratory problems
- React with unburnt hydrocarbons to form photochemical smog
- NO₂ contributes to ground-level ozone formation
NOx gases damage vegetation and aquatic ecosystems.
Unburnt Hydrocarbons
Unburnt hydrocarbons are produced when alkanes do not combust fully.
- Contribute to photochemical smog
- React with NOx in sunlight
- Can be carcinogenic
- Irritate eyes and respiratory system
Catalytic Removal of Pollutants
Modern vehicles are fitted with catalytic converters to reduce harmful emissions.
Functions of a Catalytic Converter
- Oxidises carbon monoxide to carbon dioxide
- Oxidises unburnt hydrocarbons to carbon dioxide and water
- Reduces oxides of nitrogen to nitrogen and oxygen
Example reactions:
\( \mathrm{2CO + O_2 \rightarrow 2CO_2} \)
\( \mathrm{2NO \rightarrow N_2 + O_2} \)
Platinum, palladium, and rhodium are commonly used as catalysts.
Example
State one harmful effect of carbon monoxide.
▶️ Answer / Explanation
It reduces the oxygen-carrying capacity of blood.
Example
Explain how oxides of nitrogen are formed in car engines.
▶️ Answer / Explanation
High temperatures cause nitrogen and oxygen in air to react.
This forms nitrogen oxides.
Example
Explain how catalytic converters reduce the environmental impact of car exhausts.
▶️ Answer / Explanation
They oxidise carbon monoxide and unburnt hydrocarbons.
They reduce oxides of nitrogen to nitrogen.
This lowers toxic and acidic emissions.

