CIE AS/A Level Chemistry 14.2 Alkenes Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 14.2 Alkenes Study Notes – New Syllabus
CIE AS/A Level Chemistry 14.2 Alkenes Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (including reagents and conditions) by which alkenes can be produced:
(a) elimination of HX from a halogenoalkane by ethanolic NaOH and heat
(b) dehydration of an alcohol, by using a heated catalyst (e.g. Al₂O₃) or a concentrated acid
(e.g. concentrated H₂SO₄)
(c) cracking of a longer chain alkanedescribe the following reactions of alkenes:
(a) the electrophilic addition of
(i) hydrogen in a hydrogenation reaction, H₂(g) and Pt/Ni catalyst and heat
(ii) steam, H₂O(g) and H₃PO₄ catalyst
(iii) a hydrogen halide, HX(g), at room temperature
(iv) a halogen, X₂
(b) the oxidation by cold dilute acidified KMnO₄ to form the diol
(c) the oxidation by hot concentrated acidified KMnO₄ leading to the rupture of the carbon–carbon
double bond and the identities of the subsequent products to determine the position of alkene
linkages in larger molecules
(d) addition polymerisation exemplified by the reactions of ethene and propenedescribe the use of aqueous bromine to show the presence of a C=C bond
describe the mechanism of electrophilic addition in alkenes, using bromine/ethene and hydrogen bromide/propene as examples
describe and explain the inductive effects of alkyl groups on the stability of primary, secondary and tertiary cations formed during electrophilic addition (this should be used to explain Markovnikov addition)
Preparation of Alkenes
Alkenes can be prepared by reactions that involve elimination or the breaking of large hydrocarbon molecules. At A level, you must be able to recall the reagents, conditions, and type of reaction used in each method.
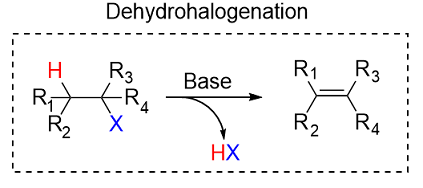
(a) Elimination of HX from a Halogenoalkane
An alkene can be formed by elimination of a hydrogen halide (HX) from a halogenoalkane.
Reagents and Conditions
- Ethanolic sodium hydroxide, \( \mathrm{NaOH} \)
- Heat under reflux
The hydroxide ion acts as a base and removes a hydrogen atom, while the halogen leaves.
Example:
\( \mathrm{CH_3CHBrCH_3 + NaOH \rightarrow CH_3CH{=}CH_2 + NaBr + H_2O} \)
This is an elimination reaction.
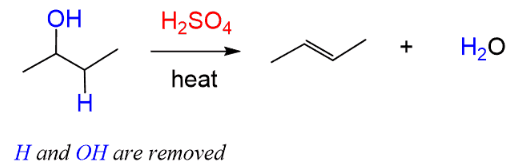
(b) Dehydration of an Alcohol
Dehydration is an elimination reaction in which water is removed from an alcohol to form an alkene.
Reagents and Conditions
- Heated aluminium oxide catalyst, \( \mathrm{Al_2O_3} \)
- OR concentrated sulfuric acid, \( \mathrm{H_2SO_4} \)
- High temperature (around 170 °C)
Example:
\( \mathrm{CH_3CH_2OH \rightarrow CH_2{=}CH_2 + H_2O} \)
The alcohol loses H and OH from adjacent carbon atoms.
(c) Cracking of Longer-Chain Alkanes
Cracking is the process in which long-chain alkanes are broken down into smaller alkanes and alkenes.

Reagents and Conditions
- High temperature
- Catalyst such as aluminium oxide, \( \mathrm{Al_2O_3} \)
- No oxygen present
Cracking always produces at least one alkene.
Example:
\( \mathrm{C_{10}H_{22} \rightarrow C_8H_{18} + C_2H_4} \)
Example
State the reagents needed to convert a halogenoalkane into an alkene.
▶️ Answer / Explanation
Ethanolic sodium hydroxide and heat.
Example
Name the type of reaction that converts ethanol into ethene.
▶️ Answer / Explanation
Elimination (dehydration).
Example
Explain why cracking of long-chain alkanes always produces an alkene.
▶️ Answer / Explanation
Breaking C–C bonds produces fragments with fewer hydrogen atoms.
This results in the formation of at least one C=C double bond.
Reactions of Alkenes
Alkenes are more reactive than alkanes because they contain a C=C double bond. The reactions of alkenes mainly involve the \( \pi \) bond and are typically addition or oxidation reactions.
(a) Electrophilic Addition Reactions of Alkenes
In electrophilic addition, the electron-rich \( \pi \) bond in the alkene attracts an electrophile. The \( \pi \) bond breaks and new \( \sigma \) bonds form.
(i) Hydrogenation
Hydrogenation is the addition of hydrogen across a C=C double bond to form an alkane.
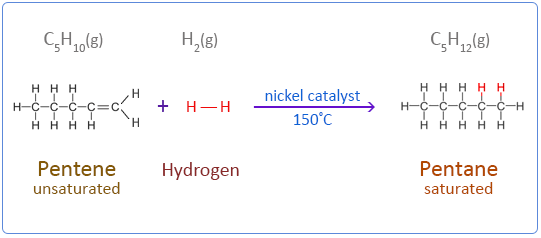
- Reagent: \( \mathrm{H_2(g)} \)
- Catalyst: Ni or Pt
- Condition: heat
\( \mathrm{CH_2{=}CH_2 + H_2 \rightarrow CH_3CH_3} \)
(ii) Hydration (Addition of Steam)
Hydration is the addition of steam across a C=C double bond to form an alcohol.

- Reagent: steam, \( \mathrm{H_2O(g)} \)
- Catalyst: phosphoric acid, \( \mathrm{H_3PO_4} \)
- Condition: high temperature and pressure
\( \mathrm{CH_2{=}CH_2 + H_2O \rightarrow CH_3CH_2OH} \)
(iii) Addition of a Hydrogen Halide
A hydrogen halide adds across the C=C bond to form a halogenoalkane.
- Reagent: HX (e.g. HBr, HCl)
- Condition: room temperature
\( \mathrm{CH_2{=}CH_2 + HBr \rightarrow CH_3CH_2Br} \)
(iv) Addition of a Halogen
Halogens add across the C=C bond to form a dihalogenoalkane.

- Reagent: \( \mathrm{Br_2} \) or \( \mathrm{Cl_2} \)
- Condition: room temperature
- Observation: bromine water is decolourised
\( \mathrm{CH_2{=}CH_2 + Br_2 \rightarrow CH_2BrCH_2Br} \)
(b) Oxidation with Cold, Dilute Acidified \( \mathrm{KMnO_4} \)
Cold, dilute acidified potassium manganate(VII) oxidises an alkene to form a vicinal diol.

- Purple solution decolourises
- Used as a test for alkenes
\( \mathrm{CH_2{=}CH_2 \rightarrow HOCH_2CH_2OH} \)
(c) Oxidation with Hot, Concentrated Acidified \( \mathrm{KMnO_4} \)
Hot, concentrated acidified potassium manganate(VII) causes oxidative cleavage of the C=C bond.
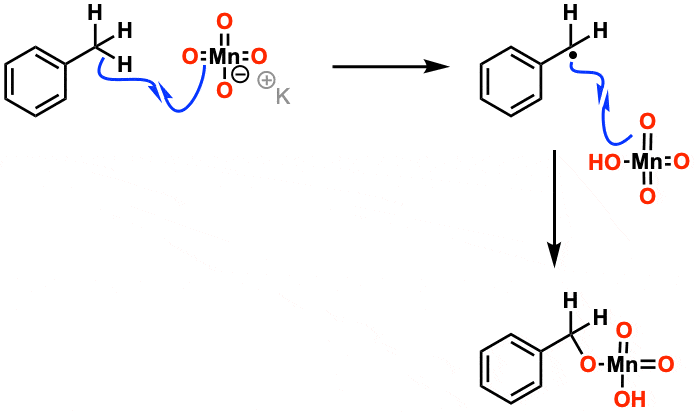
- The double bond breaks completely
- Products depend on the groups attached to the double bond
- Used to determine the position of the C=C bond
For example:
\( \mathrm{CH_3CH{=}CH_2 \rightarrow CH_3COOH + CO_2} \)
(d) Addition Polymerisation
In addition polymerisation, many alkene molecules join together to form a long-chain polymer. No small molecule is lost.
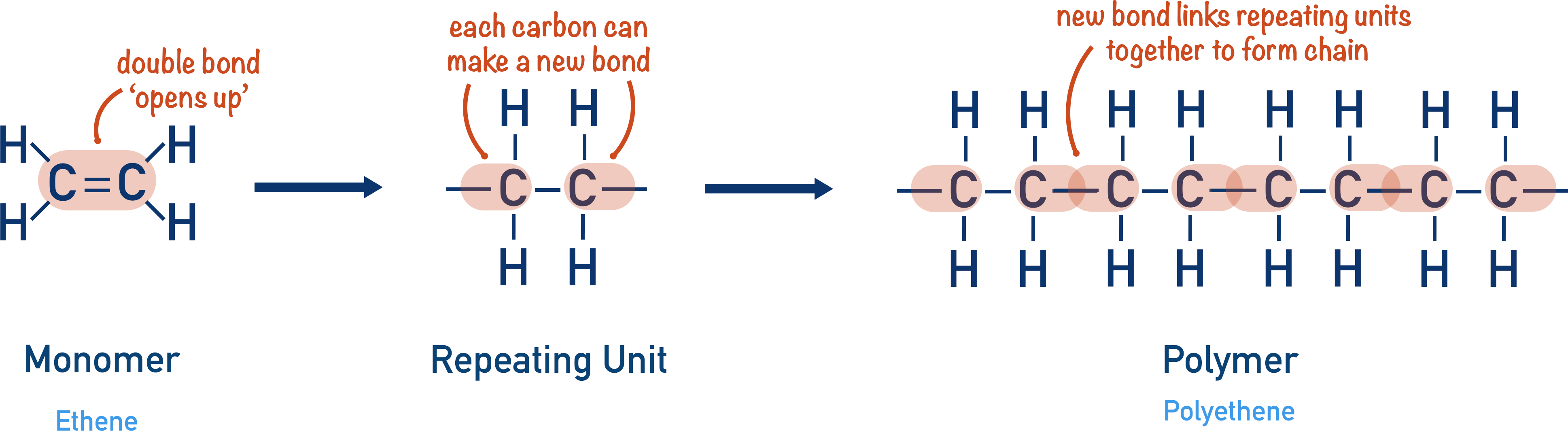
Example: Ethene
\( \mathrm{nCH_2{=}CH_2 \rightarrow (–CH_2–CH_2–)_n} \)
Example: Propene
\( \mathrm{nCH_2{=}CHCH_3 \rightarrow (–CH_2–CH(CH_3)–)_n} \)
Example
State the reagent and observation when an alkene reacts with bromine water.
▶️ Answer / Explanation
Bromine water is decolourised.
Example
Name the product formed when ethene reacts with steam in the presence of phosphoric acid.
▶️ Answer / Explanation
Ethanol.
Example
An alkene produces two carboxylic acids on oxidation with hot concentrated \( \mathrm{KMnO_4} \). Explain what this shows about the alkene structure.
▶️ Answer / Explanation
The C=C bond is internal.
Both carbon atoms of the double bond are bonded to carbon-containing groups.
Test for a C=C Double Bond using Aqueous Bromine
Aqueous bromine (often called bromine water) is used to test for the presence of a carbon–carbon double bond in an organic compound. This test is commonly used to distinguish alkenes from alkanes.
Reagent
Aqueous bromine, \( \mathrm{Br_2(aq)} \), which is orange or brown in colour.
Observation
If a C=C bond is present:
- The orange/brown colour of bromine water is decolourised
- The solution becomes colourless
If no C=C bond is present:
- No colour change is observed
- Bromine water remains orange/brown
Explanation
The \( \pi \) bond in a C=C double bond is electron-rich.
Bromine molecules are attracted to this region and undergo an electrophilic addition reaction.
The \( \pi \) bond breaks and bromine adds across the double bond, removing bromine from solution.
As bromine is consumed, the solution becomes colourless.
Example Reaction
Ethene reacting with bromine water:
\( \mathrm{CH_2{=}CH_2 + Br_2 \rightarrow CH_2BrCH_2Br} \)
Example
State the observation when bromine water is added to an alkene.
▶️ Answer / Explanation
The bromine water is decolourised.
Example
Explain why bromine water does not change colour when added to an alkane at room temperature.
▶️ Answer / Explanation
Alkanes do not contain a C=C double bond.
They do not undergo electrophilic addition with bromine water.
Example
An unknown hydrocarbon decolourises bromine water but does not react with UV light. Deduce the type of hydrocarbon present.
▶️ Answer / Explanation
The compound is an alkene.
Decolourisation shows a C=C bond, and lack of UV reaction rules out an alkane.
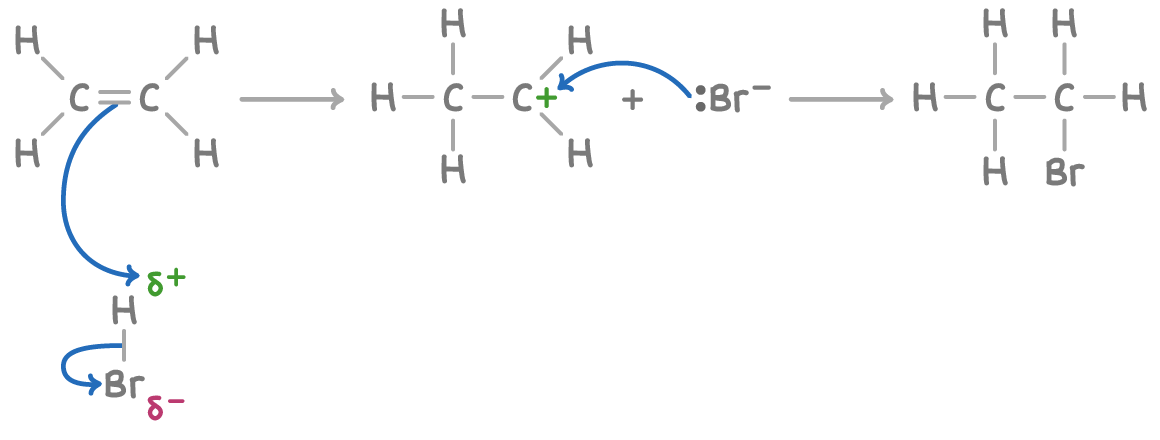
Mechanism of Electrophilic Addition in Alkenes
Electrophilic addition is the main reaction mechanism of alkenes. It occurs because the \( \pi \) bond in a C=C double bond is electron-rich and can attract electrophiles. At A level, you must be able to describe this mechanism clearly using curly arrows.
Key Ideas
- The C=C double bond contains a \( \pi \) bond with high electron density
- An electrophile is attracted to this electron-rich region
- The \( \pi \) bond breaks and two new \( \sigma \) bonds form
- The reaction proceeds in steps via a carbocation intermediate
Example 1: Bromine and Ethene
Ethene reacts with bromine by electrophilic addition to form 1,2-dibromoethane.
Overall equation:
\( \mathrm{CH_2{=}CH_2 + Br_2 \rightarrow CH_2BrCH_2Br} \)
Step 1: Polarisation of Bromine
The electron-rich \( \pi \) bond repels electrons in the bromine molecule.
This induces a dipole in \( \mathrm{Br_2} \), producing \( \mathrm{Br^{\delta+}} \) and \( \mathrm{Br^{\delta-}} \).
Step 2: Attack by the Electrophile
A curly arrow is drawn from the \( \pi \) bond to the \( \mathrm{Br^{\delta+}} \).
The Br–Br bond breaks heterolytically, forming a carbocation and a bromide ion.
Step 3: Nucleophilic Attack
The \( \mathrm{Br^-} \) ion attacks the carbocation.
A second curly arrow is drawn from the bromide lone pair to the positively charged carbon.
Example 2: Hydrogen Bromide and Propene
Propene reacts with hydrogen bromide to form a halogenoalkane. This reaction demonstrates Markovnikov’s rule.
Overall equation:
\( \mathrm{CH_3CH{=}CH_2 + HBr \rightarrow CH_3CHBrCH_3} \)
Step 1: Attack by the Electrophile (H⁺)
The \( \pi \) bond attacks the hydrogen ion, \( \mathrm{H^+} \).
A curly arrow is drawn from the \( \pi \) bond to the H atom.
The hydrogen attaches to the carbon that forms the more stable carbocation.
Step 2: Formation of the Carbocation
A secondary carbocation forms instead of a primary carbocation because it is more stable.
Step 3: Nucleophilic Attack by Br⁻
The bromide ion attacks the carbocation.
A curly arrow is drawn from the bromide lone pair to the positively charged carbon.
Key Exam Points
- Always start curly arrows from a bond or lone pair
- The electrophile attacks first
- A carbocation intermediate is formed
- The most stable carbocation is favoured
- Use correct charges and arrow directions
Example
What part of an alkene acts as a nucleophile in electrophilic addition?
▶️ Answer / Explanation
The \( \pi \) bond.
Example
Explain why a carbocation intermediate forms during electrophilic addition.
▶️ Answer / Explanation
The \( \pi \) bond breaks heterolytically.
This leaves a positively charged carbon atom.
Example
Explain why the major product formed in the reaction between propene and HBr is 2-bromopropane.
▶️ Answer / Explanation
Formation of a secondary carbocation is favoured.
Secondary carbocations are more stable than primary carbocations.
Inductive Effects and Carbocation Stability in Electrophilic Addition
During electrophilic addition to alkenes, a carbocation intermediate is formed. The stability of this carbocation strongly influences which product is formed. At A level, this must be explained using the inductive effects of alkyl groups and applied to Markovnikov addition.
Inductive Effect of Alkyl Groups
An alkyl group has a positive inductive effect (+I).

This means it pushes electron density through sigma (\( \sigma \)) bonds towards an electron-deficient centre.
- Alkyl groups donate electron density
- This reduces the positive charge on a carbocation
- The carbocation becomes more stable
Carbocation Stability
Carbocations are classified based on the number of alkyl groups attached to the positively charged carbon.
- Primary (1°) carbocation – attached to one alkyl group
- Secondary (2°) carbocation – attached to two alkyl groups
- Tertiary (3°) carbocation – attached to three alkyl groups
Stability order:
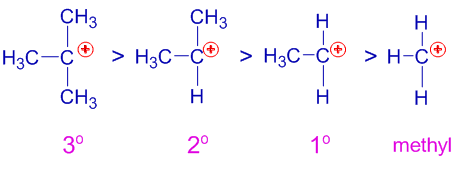
tertiary > secondary > primary
This order is due to the increasing number of alkyl groups donating electron density by the inductive effect.
Application to Electrophilic Addition
In electrophilic addition, the first step is attack by the electrophile (usually \( \mathrm{H^+} \)), forming a carbocation.
The reaction proceeds via the most stable carbocation, because this requires the lowest activation energy.
Markovnikov’s Rule Explained
Markovnikov’s rule states:
When an unsymmetrical alkene reacts with HX, the hydrogen atom adds to the carbon already bonded to the greater number of hydrogen atoms.
This can be explained using carbocation stability.
Example: Propene reacting with hydrogen bromide:
\( \mathrm{CH_3CH{=}CH_2 + HBr \rightarrow CH_3CHBrCH_3} \)
If hydrogen adds to the terminal carbon, a secondary carbocation is formed.
If hydrogen adds to the middle carbon, a primary carbocation is formed.
The secondary carbocation is more stable due to two alkyl groups donating electron density, so this pathway is favoured.
Example
State which carbocation is more stable: primary or tertiary.
▶️ Answer / Explanation
A tertiary carbocation.
Example
Explain why a secondary carbocation is more stable than a primary carbocation.
▶️ Answer / Explanation
It has more alkyl groups.
These donate electron density by the inductive effect.
Example
Explain why the reaction between but-1-ene and HBr mainly forms 2-bromobutane.
▶️ Answer / Explanation
The reaction forms a secondary rather than a primary carbocation.
The secondary carbocation is more stable due to inductive effects of alkyl groups.
This leads to Markovnikov addition.

