CIE AS/A Level Chemistry 15.1 Halogenoalkanes Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 15.1 Halogenoalkanes Study Notes – New Syllabus
CIE AS/A Level Chemistry 15.1 Halogenoalkanes Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which halogenoalkanes can be produced:
(a) the free-radical substitution of alkanes by Cl₂ or Br₂ in the presence of ultraviolet light, as exemplified by the reactions of ethane
(b) electrophilic addition of an alkene with a halogen, X₂, or hydrogen halide, HX(g), at room temperature
(c) substitution of an alcohol, e.g. by reaction with HX(g); or with KCl and concentrated H₂SO₄ or
concentrated H₃PO₄; or with PCl₃ and heat; or with PCl₅; or with SOCl₂classify halogenoalkanes into primary, secondary and tertiary
describe the following nucleophilic substitution reactions:
(a) the reaction with NaOH(aq) and heat to produce an alcohol
(b) the reaction with KCN in ethanol and heat to produce a nitrile
(c) the reaction with NH₃ in ethanol heated under pressure to produce an amine
(d) the reaction with aqueous silver nitrate in ethanol as a method of identifying the halogen present as exemplified by bromoethanedescribe the elimination reaction with NaOH in ethanol and heat to produce an alkene as exemplified by bromoethane
describe the SN1 and SN2 mechanisms of nucleophilic substitution in halogenoalkanes including the inductive effects of alkyl groups
recall that primary halogenoalkanes tend to react via the SN2 mechanism; tertiary halogenoalkanes via the SN1 mechanism; and secondary halogenoalkanes by a mixture of the two, depending on structure
describe and explain the different reactivities of halogenoalkanes (with particular reference to the relative strengths of the C–X bonds as exemplified by the reactions of halogenoalkanes with aqueous silver nitrates)
Preparation of Halogenoalkanes
Halogenoalkanes can be prepared by several reactions at AS / A level. You must be able to recall the reagents and conditions and identify the type of reaction involved.
(a) Free-Radical Substitution of Alkanes
Alkanes react with chlorine or bromine in a free-radical substitution reaction.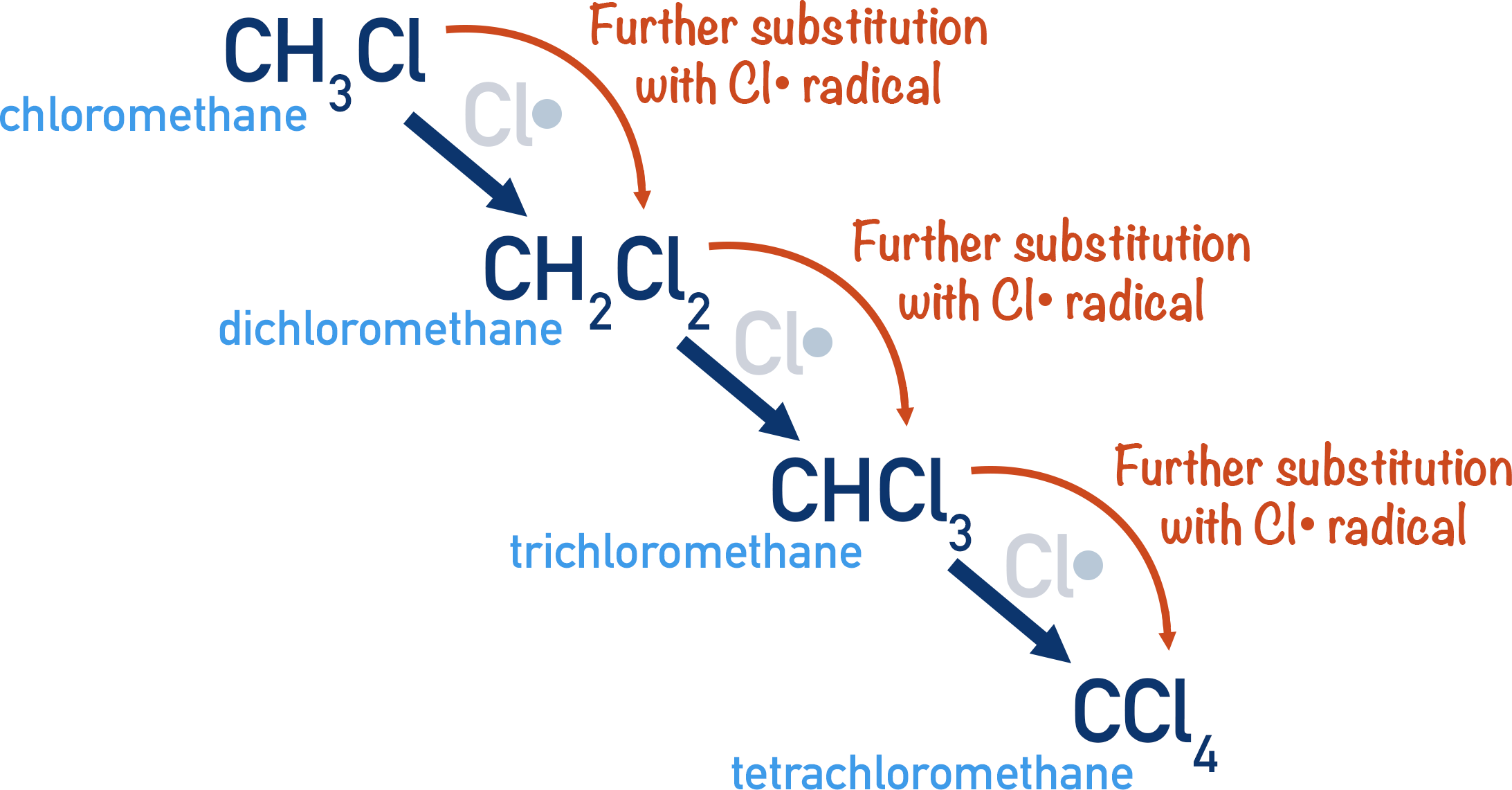
Reagents and conditions: \( \mathrm{Cl_2} \) or \( \mathrm{Br_2} \), ultraviolet light.
Example (ethane):
\( \mathrm{C_2H_6 + Cl_2 \rightarrow C_2H_5Cl + HCl} \)
This reaction is not selective and can produce a mixture of products.
(b) Electrophilic Addition to Alkenes
Alkenes form halogenoalkanes by electrophilic addition.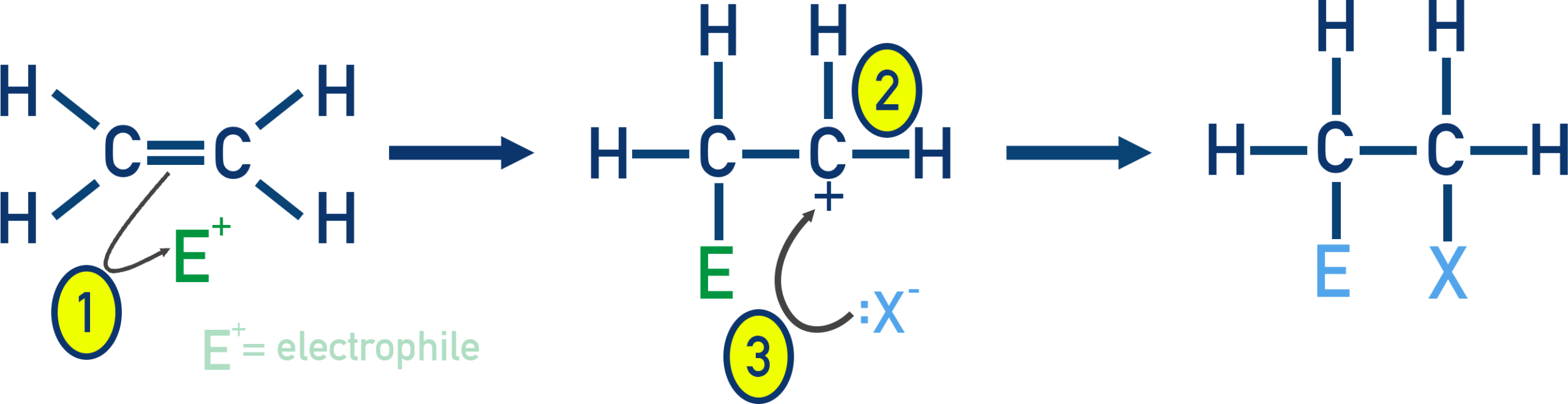
(i) Addition of halogens
Reagents and conditions: \( \mathrm{X_2} \) (X = Cl or Br), room temperature.
\( \mathrm{CH_2=CH_2 + Br_2 \rightarrow BrCH_2CH_2Br} \)
(ii) Addition of hydrogen halides
Reagents and conditions: \( \mathrm{HX(g)} \), room temperature.
\( \mathrm{CH_2=CH_2 + HBr \rightarrow CH_3CH_2Br} \)
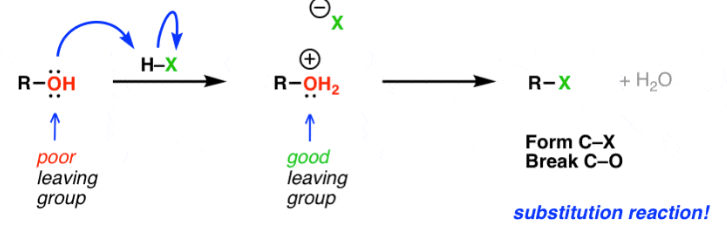
(c) Substitution of Alcohols
Alcohols can be converted into halogenoalkanes by substitution, in which the –OH group is replaced by a halogen atom.
Methods include:
- \( \mathrm{HX(g)} \)
- \( \mathrm{KCl + concentrated\ H_2SO_4} \) or \( \mathrm{concentrated\ H_3PO_4} \)
- \( \mathrm{PCl_3} \) and heat
- \( \mathrm{PCl_5} \)
- \( \mathrm{SOCl_2} \)
General equation:
\( \mathrm{ROH + HX \rightarrow RX + H_2O} \)
Examples:
\( \mathrm{ROH + PCl_5 \rightarrow RCl + POCl_3 + HCl} \)
\( \mathrm{ROH + SOCl_2 \rightarrow RCl + SO_2 + HCl} \)
Thionyl chloride is often preferred because the by-products are gases.
Example
State the reagents and conditions needed to prepare bromoethane from ethene.
▶️ Answer / Explanation
Ethene reacts with hydrogen bromide gas at room temperature.
The reaction is an electrophilic addition.
Bromoethane is formed.
Example
Describe how chloromethane can be prepared starting from methane, and explain one disadvantage of this method.
▶️ Answer / Explanation
Methane reacts with chlorine in ultraviolet light in a free-radical substitution reaction.
Chloromethane and hydrogen chloride are formed.
A disadvantage is that further substitution can occur, producing a mixture of products.
Classification of Halogenoalkanes
Halogenoalkanes are classified as primary, secondary or tertiary according to the number of carbon atoms attached to the carbon bonded to the halogen atom.
![]()
Key Rule for Classification
Identify the carbon atom directly bonded to the halogen, then count how many other carbon atoms are attached to this carbon.
Primary (1°) Halogenoalkanes
In a primary halogenoalkane, the carbon bonded to the halogen is attached to one other carbon atom.
General structure:
\( \mathrm{RCH_2X} \)
Example: bromoethane, \( \mathrm{CH_3CH_2Br} \)
Secondary (2°) Halogenoalkanes
In a secondary halogenoalkane, the carbon bonded to the halogen is attached to two other carbon atoms.
General structure:
\( \mathrm{R_2CHX} \)
Example: 2-bromopropane, \( \mathrm{CH_3CHBrCH_3} \)
Tertiary (3°) Halogenoalkanes
In a tertiary halogenoalkane, the carbon bonded to the halogen is attached to three other carbon atoms.
General structure:
\( \mathrm{R_3CX} \)
Example: 2-chloro-2-methylpropane
In molecules containing more than one halogen atom, each halogen-bearing carbon must be classified separately.
Example
Classify the halogenoalkane \( \mathrm{CH_3CH_2CH_2Cl} \).
▶️ Answer / Explanation
The carbon bonded to chlorine is attached to only one other carbon.
The compound is a primary halogenoalkane.
Example
A compound has the structure \( \mathrm{(CH_3)_3CCl} \). Classify the halogenoalkane and justify your answer.
▶️ Answer / Explanation
The carbon bonded to chlorine is attached to three other carbon atoms.
This corresponds to a tertiary halogenoalkane.
Nucleophilic Substitution Reactions of Halogenoalkanes
Halogenoalkanes undergo nucleophilic substitution reactions because the C–X bond is polar, making the carbon atom electron deficient and susceptible to attack by nucleophiles.
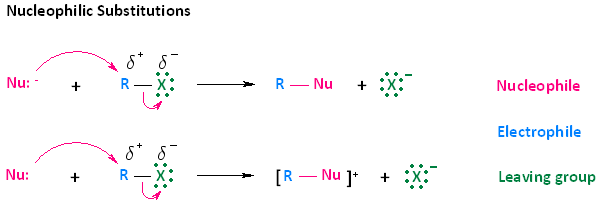
(a) Reaction with Aqueous Sodium Hydroxide
Halogenoalkanes react with aqueous hydroxide ions to form alcohols.
Reagents and conditions: \( \mathrm{NaOH(aq)} \), heat under reflux.
This is a nucleophilic substitution reaction.
General equation:
\( \mathrm{R{-}X + OH^- \rightarrow R{-}OH + X^-} \)
(b) Reaction with Potassium Cyanide
Halogenoalkanes react with cyanide ions to form nitriles.
Reagents and conditions: \( \mathrm{KCN} \) in ethanol, heat under reflux.
The cyanide ion acts as a nucleophile.
General equation:
\( \mathrm{R{-}X + CN^- \rightarrow R{-}CN + X^-} \)
This reaction increases the carbon chain length by one carbon atom.
(c) Reaction with Ammonia
Halogenoalkanes react with ammonia to form amines.
Reagents and conditions: excess \( \mathrm{NH_3} \) in ethanol, heated under pressure.
General equation:
\( \mathrm{R{-}X + NH_3 \rightarrow R{-}NH_2 + HX} \)
Using excess ammonia helps to reduce further substitution reactions.
(d) Reaction with Aqueous Silver Nitrate
Halogenoalkanes can be identified by reaction with aqueous silver nitrate in ethanol.
The halogenoalkane first undergoes hydrolysis, releasing halide ions, which then react with silver ions.
Example (bromoethane):
\( \mathrm{C_2H_5Br + H_2O \rightarrow C_2H_5OH + H^+ + Br^-} \)
\( \mathrm{Ag^+ + Br^- \rightarrow AgBr(s)} \)
Observations
- Chloride → white precipitate, \( \mathrm{AgCl} \)
- Bromide → cream precipitate, \( \mathrm{AgBr} \)
- Iodide → yellow precipitate, \( \mathrm{AgI} \)
The rate of precipitation increases from chloroalkanes to iodoalkanes.
Example
State the product formed when bromoethane is heated with aqueous sodium hydroxide.
▶️ Answer / Explanation
Bromoethane undergoes nucleophilic substitution.
The bromine atom is replaced by a hydroxyl group.
The product is ethanol.
Example
Describe how aqueous silver nitrate in ethanol can be used to distinguish between chloroethane and bromoethane.
▶️ Answer / Explanation
Both compounds undergo hydrolysis to release halide ions.
Chloroethane forms a white precipitate of \( \mathrm{AgCl} \).
Bromoethane forms a cream precipitate of \( \mathrm{AgBr} \).
The bromide precipitate also forms more rapidly.
Elimination Reaction of Halogenoalkanes
Halogenoalkanes can undergo elimination reactions to form alkenes. This reaction competes with nucleophilic substitution and occurs under specific conditions.
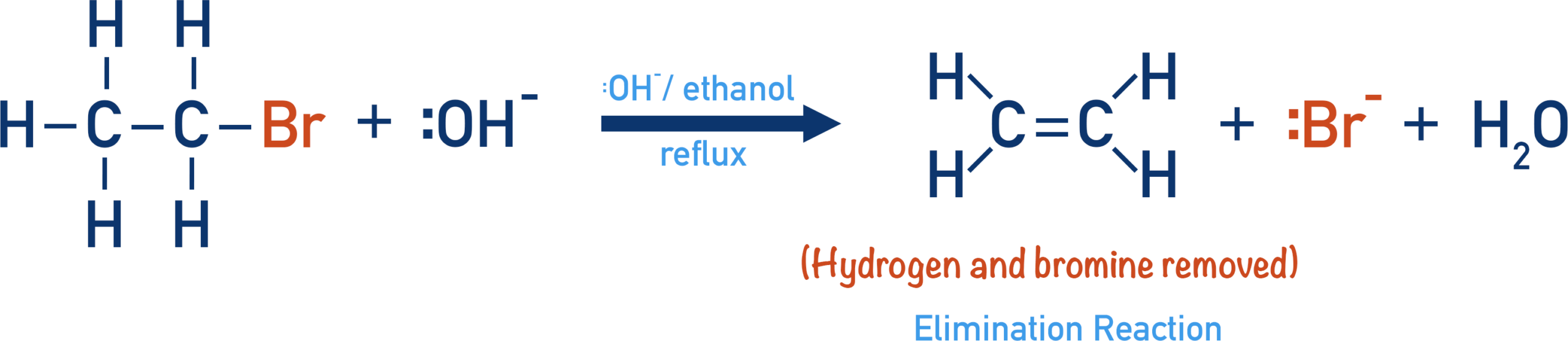
Reaction with Ethanolic Sodium Hydroxide
When a halogenoalkane is heated with sodium hydroxide dissolved in ethanol, an elimination reaction occurs.
Reagents and conditions: \( \mathrm{NaOH} \) in ethanol, heat under reflux.
What is Eliminated?
A hydrogen atom is removed from a carbon adjacent to the carbon bonded to the halogen, and the halide ion is eliminated.
This results in the formation of a carbon–carbon double bond.
Example: Bromoethane
Bromoethane undergoes elimination to form ethene:
\( \mathrm{CH_3CH_2Br + OH^- \rightarrow CH_2{=}CH_2 + H_2O + Br^-} \)
Role of Ethanol
Ethanol acts as a non-aqueous solvent.
This favours elimination over substitution by reducing the availability of hydroxide ions as nucleophiles.
Key Features of Elimination
- Produces an alkene
- Requires heat
- Favoured by ethanolic NaOH
- Occurs alongside substitution, but conditions favour elimination
Example
State the product formed when bromoethane is heated with sodium hydroxide in ethanol.
▶️ Answer / Explanation
Bromoethane undergoes elimination.
Hydrogen bromide is eliminated from the molecule.
The product formed is ethene.
Example
Explain why ethanolic sodium hydroxide produces an alkene from a halogenoalkane, whereas aqueous sodium hydroxide produces an alcohol.
▶️ Answer / Explanation
Ethanol reduces the nucleophilicity of hydroxide ions.
This favours elimination rather than substitution.
Heat promotes the loss of hydrogen halide.
Aqueous sodium hydroxide favours substitution to form an alcohol.
\(\mathrm{S_N1}\) and \(\mathrm{S_N2}\) Mechanisms of Nucleophilic Substitution
Halogenoalkanes undergo nucleophilic substitution by two different mechanisms: \(\mathrm{S_N1}\) and \(\mathrm{S_N2}\). The mechanism followed depends on the structure of the halogenoalkane and the stability of intermediates.
Polarity of the C–X Bond
In halogenoalkanes, the C–X bond is polar.
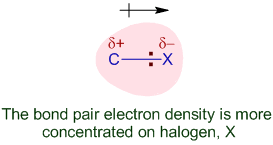
The halogen is more electronegative than carbon, making the carbon atom electron deficient and susceptible to nucleophilic attack.
\(\mathrm{S_N2}\) Mechanism
The \(\mathrm{S_N2}\) mechanism occurs in one step. The nucleophile attacks the carbon bonded to the halogen at the same time as the C–X bond breaks.

General equation:
\( \mathrm{R{-}X + OH^- \rightarrow R{-}OH + X^-} \)
Factors Affecting \(\mathrm{S_N2}\)
- Favoured by primary halogenoalkanes
- Occurs fastest when there is little steric hindrance
- The nucleophile must be able to approach the carbon easily
Primary halogenoalkanes react mainly by \(\mathrm{S_N2}\) because alkyl groups are small and do not block the nucleophile.
\(\mathrm{S_N1}\) Mechanism
The \(\mathrm{S_N1}\) mechanism occurs in two steps.

Step 1: The C–X bond breaks heterolytically to form a carbocation.
\( \mathrm{R{-}X \rightarrow R^+ + X^-} \)
Step 2: The nucleophile attacks the carbocation.
\( \mathrm{R^+ + OH^- \rightarrow R{-}OH} \)
Role of Inductive Effects
Alkyl groups have a positive inductive effect.
They donate electron density towards the positively charged carbon atom, stabilising the carbocation.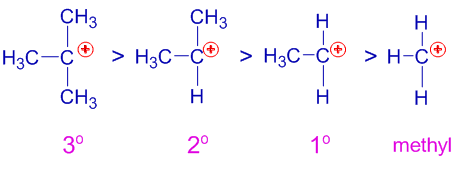
As a result:
- Tertiary carbocations are most stable
- Secondary carbocations are moderately stable
- Primary carbocations are very unstable
Therefore:
- Tertiary halogenoalkanes react mainly by \(\mathrm{S_N1}\)
- Primary halogenoalkanes react mainly by \(\mathrm{S_N2}\)
- Secondary halogenoalkanes can react by either mechanism
Comparison Summary
- \(\mathrm{S_N1}\) → two-step mechanism with carbocation intermediate
- \(\mathrm{S_N2}\) → one-step mechanism with no intermediate
- Inductive effects stabilise carbocations in \(\mathrm{S_N1}\)
- Steric hindrance slows \(\mathrm{S_N2}\) reactions
Example
Explain why tertiary halogenoalkanes undergo nucleophilic substitution mainly by the \(\mathrm{S_N1}\) mechanism.
▶️ Answer / Explanation
Tertiary halogenoalkanes form relatively stable carbocations.
Alkyl groups donate electron density by a positive inductive effect.
This stabilises the carbocation, allowing the \(\mathrm{S_N1}\) mechanism to occur.
Example
Explain why primary halogenoalkanes react more slowly by \(\mathrm{S_N1}\) than by \(\mathrm{S_N2}\).
▶️ Answer / Explanation
Primary carbocations are very unstable.
They are not stabilised sufficiently by alkyl groups.
Formation of a carbocation is therefore unfavourable.
Primary halogenoalkanes instead react by the one-step \(\mathrm{S_N2}\) mechanism.
Nucleophilic Substitution Mechanisms and Halogenoalkane Type
The mechanism by which a halogenoalkane undergoes nucleophilic substitution depends on whether it is primary, secondary or tertiary. This is determined by carbocation stability and steric hindrance.
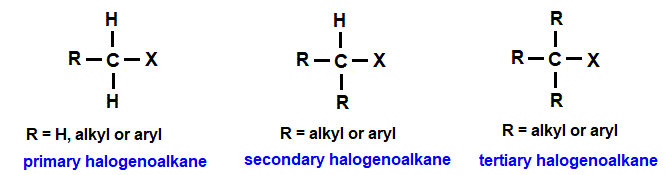
Primary Halogenoalkanes
Primary halogenoalkanes react mainly by the SN2 mechanism.
- Carbocations would be very unstable
- Little steric hindrance allows nucleophilic attack
- Reaction occurs in one step
Secondary Halogenoalkanes
Secondary halogenoalkanes react by a mixture of SN1 and SN2 mechanisms.
- Carbocations have moderate stability
- Steric hindrance partially restricts SN2 attack
- The dominant mechanism depends on structure and conditions
Tertiary Halogenoalkanes
Tertiary halogenoalkanes react mainly by the SN1 mechanism.
- Carbocations are stabilised by alkyl groups
- Strong positive inductive effect stabilises the intermediate
- Severe steric hindrance prevents SN2 attack
Primary halogenoalkanes react mainly by SN2, tertiary halogenoalkanes react mainly by SN1, and secondary halogenoalkanes react by a mixture of both mechanisms, depending on their structure.
Example
State the most likely nucleophilic substitution mechanism for 1-bromopropane and give one reason.
▶️ Answer / Explanation
1-bromopropane is a primary halogenoalkane.
Primary halogenoalkanes react mainly by the SN2 mechanism.
This is because a primary carbocation would be very unstable.
Example
2-bromopropane undergoes nucleophilic substitution by more than one mechanism. Explain why.
▶️ Answer / Explanation
2-bromopropane is a secondary halogenoalkane.
The secondary carbocation has moderate stability.
Steric hindrance partially restricts SN2 attack.
As a result, both SN1 and SN2 mechanisms can occur.
Reactivity of Halogenoalkanes
Halogenoalkanes show different reactivities in nucleophilic substitution reactions. These differences can be explained by the strength of the C–X bond, where X is the halogen.
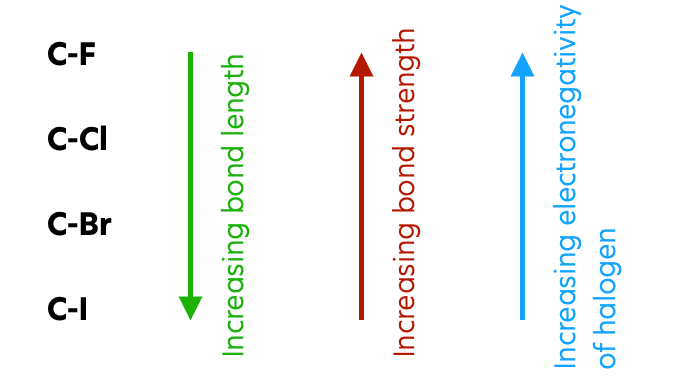
C–X Bond Strength
As the halogen atom increases in size down Group 17, the C–X bond length increases and the C–X bond strength decreases.
Order of C–X bond strength:
\( \mathrm{C{-}F > C{-}Cl > C{-}Br > C{-}I} \)
As a result, the C–I bond is the weakest and is broken most easily, while the C–Cl bond is stronger and breaks more slowly.
Effect on Reactivity
Weaker C–X bonds require less energy to break, making nucleophilic substitution faster.
Therefore, the overall reactivity order of halogenoalkanes is:
\( \mathrm{R{-}I > R{-}Br > R{-}Cl} \)
Reaction with Aqueous Silver Nitrate
Halogenoalkanes can be compared experimentally by their reaction with aqueous silver nitrate in ethanol.
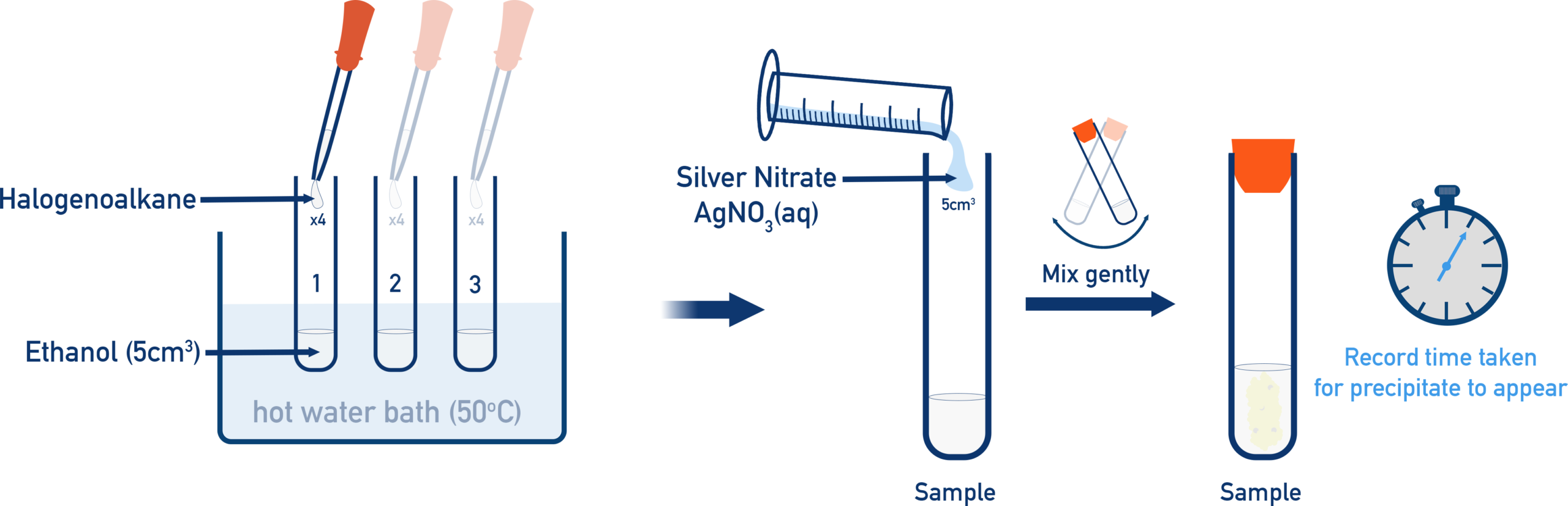
The halogenoalkane first undergoes hydrolysis, producing halide ions.
Example (bromoethane):
\( \mathrm{C_2H_5Br + H_2O \rightarrow C_2H_5OH + H^+ + Br^-} \)
The halide ions then react with silver ions:
\( \mathrm{Ag^+ + Br^- \rightarrow AgBr(s)} \)
Observations
- Chloroalkanes form a white precipitate slowly
- Bromoalkanes form a cream precipitate more quickly
- Iodoalkanes form a yellow precipitate very rapidly
The increasing speed of precipitate formation reflects the decreasing C–X bond strength.
Iodoalkanes react fastest because the C–I bond is weakest and breaks most easily. Chloroalkanes react slowest because the C–Cl bond is stronger and requires more energy to break.
Example
Explain why bromoethane reacts faster than chloroethane with aqueous silver nitrate.
▶️ Answer / Explanation
The C–Br bond is weaker than the C–Cl bond.
Less energy is required to break the C–Br bond.
Bromide ions are released more quickly.
This causes the silver bromide precipitate to form faster.
Example
Iodoethane produces a yellow precipitate almost immediately when warmed with aqueous silver nitrate, while chloroethane reacts slowly. Explain this difference in reactivity.
▶️ Answer / Explanation
The C–I bond in iodoethane is significantly weaker than the C–Cl bond in chloroethane.
This bond breaks more readily, allowing faster hydrolysis.
Iodide ions are released quickly and react immediately with silver ions.
Chloroethane reacts more slowly because the stronger C–Cl bond is harder to break.
