CIE AS/A Level Chemistry 16.1 Alcohols Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 16.1 Alcohols Study Notes – New Syllabus
CIE AS/A Level Chemistry 16.1 Alcohols Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which alcohols can be produced:
(a) electrophilic addition of steam to an alkene, H₂O(g) and H₃PO₄ catalyst
(b) reaction of alkenes with cold dilute acidified potassium manganate(VII) to form a diol
(c) substitution of a halogenoalkane using NaOH(aq) and heat
(d) reduction of an aldehyde or ketone using NaBH₄ or LiAlH₄
(e) reduction of a carboxylic acid using LiAlH₄
(f) hydrolysis of an ester using dilute acid or dilute alkali and heatdescribe:
(a) the reaction with oxygen (combustion)
(b) substitution to form halogenoalkanes, e.g. by reaction with HX(g); or with KCl and concentrated
H₂SO₄ or concentrated H₃PO₄; or with PCl₃ and heat; or with PCl₅; or with SOCl₂
(c) the reaction with Na(s)
(d) oxidation with acidified K₂Cr₂O₇ or acidified KMnO₄ to:
(i) carbonyl compounds by distillation
(ii) carboxylic acids by refluxing
(primary alcohols give aldehydes which can be further oxidised to carboxylic acids, secondary
alcohols give ketones, tertiary alcohols cannot be oxidised)
(e) dehydration to an alkene, by using a heated catalyst, e.g. Al₂O₃ or a concentrated acid
(f) formation of esters by reaction with carboxylic acids and concentrated H₂SO₄ as catalyst as exemplified by ethanol(a) classify alcohols as primary, secondary and tertiary alcohols, to include examples with more than one alcohol group
(b) state characteristic distinguishing reactions, e.g. mild oxidation with acidified K₂Cr₂O₇, colour change from orange to greendeduce the presence of a CH₃CH(OH)– group in an alcohol, CH₃CH(OH)–R, from its reaction with alkaline I₂(aq) to form a yellow precipitate of tri-iodomethane and an ion, RCO₂⁻
explain the acidity of alcohols compared with water
Preparation of Alcohols
Alcohols can be prepared by a variety of reactions at AS / A level. It is essential to recall the reagents and conditions for each method.
(a) Electrophilic Addition of Steam to an Alkene
Alkenes react with steam to form alcohols by electrophilic addition.
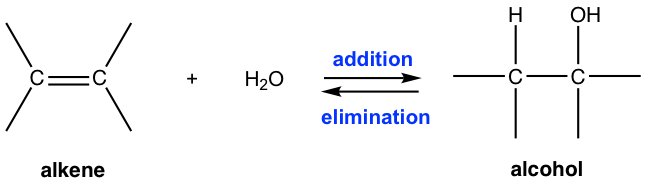
Reagents and conditions: \( \mathrm{H_2O(g)} \), phosphoric acid catalyst, high temperature and pressure.
General equation:
\( \mathrm{CH_2=CH_2 + H_2O \rightarrow CH_3CH_2OH} \)
(b) Oxidation of Alkenes with Potassium Manganate(VII)
Alkenes react with cold, dilute, acidified potassium manganate(VII) to form diols.

Reagents and conditions: cold, dilute, acidified \( \mathrm{KMnO_4} \).
General equation (ethene):
\( \mathrm{CH_2=CH_2 \rightarrow HOCH_2CH_2OH} \)
(c) Substitution of a Halogenoalkane
Halogenoalkanes react with aqueous hydroxide ions in a nucleophilic substitution reaction to form alcohols.
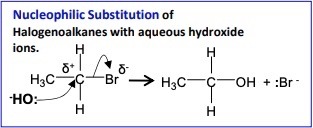
Reagents and conditions: \( \mathrm{NaOH(aq)} \), heat under reflux.
General equation:
\( \mathrm{R{-}X + OH^- \rightarrow R{-}OH + X^-} \)
(d) Reduction of Aldehydes and Ketones
Aldehydes and ketones can be reduced to alcohols using sodium borohydride or lithium aluminium hydride.
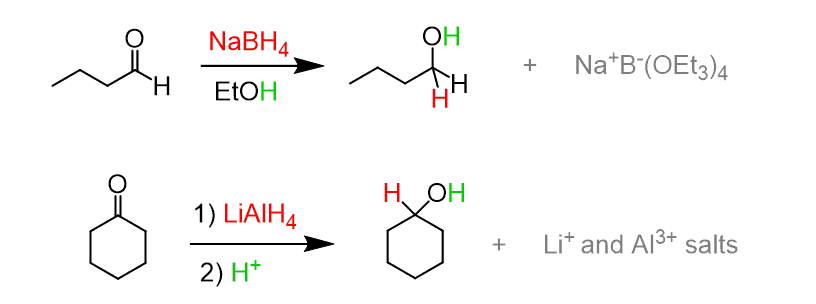
Reagents and conditions: \( \mathrm{NaBH_4} \) or \( \mathrm{LiAlH_4} \).
General equations:
\( \mathrm{RCHO \rightarrow RCH_2OH} \)
\( \mathrm{RCOR’ \rightarrow RCHOHR’} \)
(e) Reduction of Carboxylic Acids
Carboxylic acids can be reduced to primary alcohols using lithium aluminium hydride.

Reagents and conditions: \( \mathrm{LiAlH_4} \) in dry ether.
General equation:
\( \mathrm{RCOOH \rightarrow RCH_2OH} \)
(f) Hydrolysis of Esters
Esters can be hydrolysed to form alcohols using dilute acid or dilute alkali and heat.

Reagents and conditions: dilute acid or dilute alkali, heat under reflux.
General equation:
\( \mathrm{RCOOR’ \rightarrow R’OH} \)
Example
State the reagents and conditions required to prepare ethanol from bromoethane.
▶️ Answer / Explanation
Bromoethane is heated under reflux with aqueous sodium hydroxide.
A nucleophilic substitution reaction forms ethanol.
Example
Devise a two-step synthesis to prepare propan-1-ol from propene.
▶️ Answer / Explanation
Step 1: React propene with cold, dilute, acidified potassium manganate(VII) to form a diol.
Step 2: Reduce an appropriate intermediate or carry out controlled reactions to obtain propan-1-ol.
All reagents and conditions are from the syllabus.
Reactions of Alcohols
Alcohols undergo a range of important reactions at AS / A level. These include combustion, substitution, oxidation, dehydration, and esterification.

(a) Combustion
Alcohols burn in oxygen in an exothermic reaction to form carbon dioxide and water.
General equation:
\( \mathrm{C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O} \)
(b) Substitution to Form Halogenoalkanes
Alcohols undergo substitution reactions in which the –OH group is replaced by a halogen atom.
Methods include:
- Reaction with hydrogen halides, \( \mathrm{HX(g)} \)
- Reaction with potassium halide and concentrated acid
- Reaction with phosphorus chlorides
- Reaction with thionyl chloride
General equation:
\( \mathrm{ROH + HX \rightarrow RX + H_2O} \)
Examples:
\( \mathrm{ROH + PCl_5 \rightarrow RCl + POCl_3 + HCl} \)
\( \mathrm{ROH + SOCl_2 \rightarrow RCl + SO_2 + HCl} \)
(c) Reaction with Sodium
Alcohols react with sodium metal to form a sodium alkoxide and hydrogen gas.
General equation:
\( \mathrm{2ROH + 2Na \rightarrow 2RONa + H_2} \)
(d) Oxidation of Alcohols
(i) Oxidation to Carbonyl Compounds
Primary and secondary alcohols can be oxidised using acidified potassium dichromate(VI) or acidified potassium manganate(VII).
Conditions: heat with distillation.
Primary alcohol:
\( \mathrm{RCH_2OH \rightarrow RCHO} \)
Secondary alcohol:
\( \mathrm{R_2CHOH \rightarrow R_2CO} \)
(ii) Oxidation to Carboxylic Acids
Primary alcohols can be further oxidised to carboxylic acids.
Conditions: heat under reflux.
\( \mathrm{RCH_2OH \rightarrow RCOOH} \)
Secondary alcohols give ketones only. Tertiary alcohols cannot be oxidised.
(e) Dehydration to Form an Alkene
Alcohols can be dehydrated to form alkenes by eliminating water.
Conditions: heated \( \mathrm{Al_2O_3} \) catalyst or concentrated acid.
General equation:
\( \mathrm{CH_3CH_2OH \rightarrow CH_2=CH_2 + H_2O} \)
(f) Esterification
Alcohols react with carboxylic acids to form esters and water.
Conditions: concentrated sulfuric acid catalyst, heat under reflux.
Example (ethanol):
\( \mathrm{CH_3CH_2OH + CH_3COOH \rightleftharpoons CH_3COOCH_2CH_3 + H_2O} \)
Example
Describe how ethanol reacts with sodium metal.
▶️ Answer / Explanation
Ethanol reacts with sodium to form sodium ethoxide.
Hydrogen gas is released.
The reaction shows the weakly acidic nature of ethanol.
Example
Explain how the oxidation of propan-1-ol can be controlled to form either an aldehyde or a carboxylic acid.
▶️ Answer / Explanation
Distillation removes the aldehyde as it forms, preventing further oxidation.
Reflux allows continued oxidation to the carboxylic acid.
Control of conditions determines the final product.
Classification and Identification of Alcohols
Alcohols can be classified as primary, secondary or tertiary depending on the number of carbon atoms attached to the carbon bearing the –OH group.
(a) Classification of Alcohols

Primary alcohols have the –OH group attached to a carbon bonded to one other carbon atom.
General structure:
\( \mathrm{RCH_2OH} \)
Example: ethanol, \( \mathrm{CH_3CH_2OH} \)
Secondary alcohols have the –OH group attached to a carbon bonded to two other carbon atoms.
General structure:
\( \mathrm{R_2CHOH} \)
Example: propan-2-ol, \( \mathrm{CH_3CHOHCH_3} \)
Tertiary alcohols have the –OH group attached to a carbon bonded to three other carbon atoms.
General structure:
\( \mathrm{R_3COH} \)
Example: 2-methylpropan-2-ol
In molecules containing more than one –OH group, each –OH group is classified separately.
Example: propane-1,2-diol contains
- one primary alcohol group
- one secondary alcohol group
(b) Distinguishing Reactions: Oxidation
Alcohols can be distinguished using mild oxidation with acidified potassium dichromate(VI).
Reagent: acidified \( \mathrm{K_2Cr_2O_7} \)
Observation: colour change from orange to green if oxidation occurs.
Results of the Test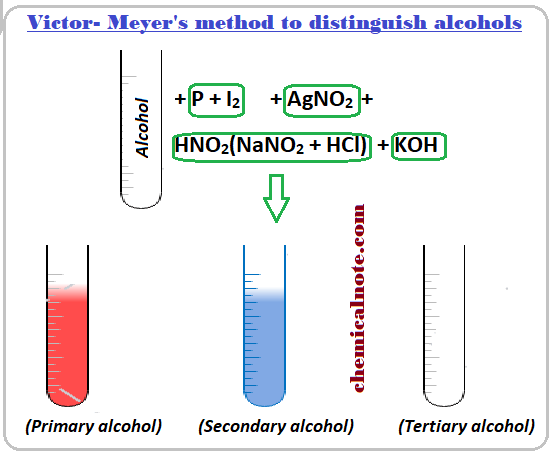
- Primary alcohol → oxidised (orange → green)
- Secondary alcohol → oxidised (orange → green)
- Tertiary alcohol → no reaction (no colour change)
Primary alcohol oxidation:
\( \mathrm{RCH_2OH \rightarrow RCHO \rightarrow RCOOH} \)
Secondary alcohol oxidation:
\( \mathrm{R_2CHOH \rightarrow R_2CO} \)
Tertiary alcohols are not oxidised under these conditions.
Example
A colour change from orange to green is observed when an alcohol is warmed with acidified potassium dichromate(VI). What types of alcohol could be present?
▶️ Answer / Explanation
The colour change indicates oxidation.
Primary and secondary alcohols are oxidised.
The alcohol cannot be tertiary.
Example
A diol reacts with acidified potassium dichromate(VI) showing an orange to green colour change. Explain what this indicates about the –OH groups present.
▶️ Answer / Explanation
The colour change shows that at least one –OH group is oxidised.
Therefore at least one of the alcohol groups is primary or secondary.
A tertiary alcohol group would not give this colour change.
Test for the CH3CH(OH)– Group: Iodoform Reaction
Alcohols containing the CH3CH(OH)– group can be identified using the iodoform test. This is a characteristic reaction of certain secondary alcohols.

Reagent
The reagent used is alkaline iodine, \( \mathrm{I_2(aq)} \) with sodium hydroxide.
Observation
A yellow precipitate of tri-iodomethane, \( \mathrm{CHI_3} \), is formed.
Meaning of a Positive Test
A positive iodoform test for an alcohol indicates the presence of a CH3CH(OH)– group, with the general structure \( \mathrm{CH_3CH(OH){-}R} \).
During the reaction, the alcohol is oxidised to a carbonyl compound, which then undergoes the iodoform reaction.
Reaction
The reaction produces tri-iodomethane and a carboxylate ion, \( \mathrm{RCO_2^-} \).
General equation:
\( \mathrm{CH_3CH(OH){-}R + 3I_2 + 4OH^- \rightarrow CHI_3 + RCO_2^- + 3I^- + 3H_2O} \)
Only alcohols containing the CH3CH(OH)– group give this reaction. Other primary and tertiary alcohols give no yellow precipitate.
Examples That Give a Positive Test
- Ethanol, \( \mathrm{CH_3CH_2OH} \)
- Propan-2-ol, \( \mathrm{CH_3CH(OH)CH_3} \)
Example
An alcohol gives a yellow precipitate when warmed with alkaline iodine. What structural feature must be present?
▶️ Answer / Explanation
The yellow precipitate is tri-iodomethane.
This indicates a positive iodoform test.
The alcohol must contain a CH3CH(OH)– group.
Example
An organic compound gives a positive 2,4-DNPH test only after oxidation and also produces a yellow precipitate with alkaline iodine. Deduce the nature of the alcohol present.
▶️ Answer / Explanation
The positive iodoform test indicates the presence of a CH3CH(OH)– group.
The need for oxidation before the 2,4-DNPH test shows the compound is an alcohol.
The alcohol must therefore be a secondary alcohol containing a CH3CH(OH)– group.
Acidity of Alcohols Compared with Water
Alcohols and water both contain an –OH group and can donate a proton. However, alcohols are generally less acidic than water.
Acid–Base Behaviour
Acidity depends on how easily a substance can lose a proton and how stable the conjugate base is.
Water:
\( \mathrm{H_2O \rightleftharpoons H^+ + OH^-} \)
Alcohol:
\( \mathrm{ROH \rightleftharpoons H^+ + RO^-} \)
Effect of the Alkyl Group
In alcohols, the alkyl group has a positive inductive effect.
This pushes electron density towards the oxygen atom, making the O–H bond less polar.
As a result, the proton is held more strongly and is less easily lost than in water.
Stability of the Conjugate Base
The conjugate base of water, \( \mathrm{OH^-} \), is more stable than the conjugate base of an alcohol, \( \mathrm{RO^-} \).
The alkyl group destabilises the negative charge on the alkoxide ion by electron donation.
Because the conjugate base of an alcohol is less stable, alcohols are weaker acids than water.
Key Conclusions
- Alcohols can donate a proton
- Alcohols are less acidic than water
- Alkyl groups donate electron density
- Alkoxide ions are less stable than hydroxide ions
Example
Explain why ethanol is less acidic than water.
▶️ Answer / Explanation
Ethanol contains an alkyl group with a positive inductive effect.
This reduces the polarity of the O–H bond.
The proton is less easily lost than in water.
The ethoxide ion is less stable than the hydroxide ion.
Example
Explain why tert-butanol is less acidic than ethanol.
▶️ Answer / Explanation
Tert-butanol contains more alkyl groups than ethanol.
These alkyl groups have a greater electron-donating inductive effect.
This further destabilises the alkoxide ion.
The O–H bond is therefore less polar and the proton is less easily lost.
