CIE AS/A Level Chemistry 17.1 Aldehydes and ketones Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 17.1 Aldehydes and ketones Study Notes – New Syllabus
CIE AS/A Level Chemistry 17.1 Aldehydes and ketones Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which aldehydes and ketones can be produced:
(a) the oxidation of primary alcohols using acidified K₂Cr₂O₇ or acidified KMnO₄ and distillation to produce aldehydes
(b) the oxidation of secondary alcohols using acidified K₂Cr₂O₇ or acidified KMnO₄ and distillation to produce ketonesdescribe:
(a) the reduction of aldehydes and ketones using NaBH₄ or LiAlH₄ to produce alcohols
(b) the reaction of aldehydes and ketones with HCN, KCN as catalyst, and heat to produce hydroxynitriles as exemplified by ethanal and propanonedescribe the mechanism of the nucleophilic addition reactions of hydrogen cyanide with aldehydes and ketones in 17.1.2(b)
describe the use of 2,4-dinitrophenylhydrazine (2,4-DNPH reagent) to detect the presence of carbonyl compounds
deduce the nature (aldehyde or ketone) of an unknown carbonyl compound from the results of simple tests (Fehling’s and Tollens’ reagents; ease of oxidation)
deduce the presence of a CH₃CO– group in an aldehyde or ketone, CH₃CO–R, from its reaction with alkaline I₂(aq) to form a yellow precipitate of tri-iodomethane and an ion, RCO₂⁻
Preparation of Aldehydes and Ketones
Aldehydes and ketones can be prepared by the oxidation of alcohols. Careful control of the reaction conditions is required to obtain the correct product.
(a) Oxidation of Primary Alcohols to Aldehydes
Primary alcohols can be oxidised to aldehydes using acidified potassium dichromate(VI) or acidified potassium manganate(VII).
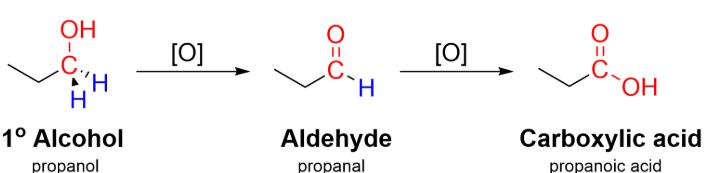
Conditions: gentle heating with distillation.
Distillation removes the aldehyde as it forms, preventing further oxidation to a carboxylic acid.
General equation: \( \mathrm{RCH_2OH \rightarrow RCHO} \)
(b) Oxidation of Secondary Alcohols to Ketones
Secondary alcohols can be oxidised to ketones using acidified potassium dichromate(VI) or acidified potassium manganate(VII).

Conditions: heat with distillation.
Further oxidation does not occur because ketones are resistant to oxidation under these conditions.
General equation: \( \mathrm{R_2CHOH \rightarrow R_2CO} \)
Primary alcohols must not be heated under reflux if an aldehyde is required, as this would produce a carboxylic acid.
Example
State the reagents and conditions needed to prepare ethanal from ethanol.
▶️ Answer / Explanation
Ethanol is oxidised using acidified potassium dichromate(VI).
The mixture is gently heated and the ethanal is distilled off as it forms.
Example
Explain why distillation is used instead of reflux when preparing an aldehyde from a primary alcohol.
▶️ Answer / Explanation
Distillation removes the aldehyde from the reaction mixture as it forms.
This prevents further oxidation of the aldehyde to a carboxylic acid.
Reflux would allow continued oxidation to occur.
Reactions of Aldehydes and Ketones
Aldehydes and ketones undergo several important reactions at AS / A level. These include reduction to alcohols and nucleophilic addition with hydrogen cyanide to form hydroxynitriles.
(a) Reduction to Alcohols
Aldehydes and ketones can be reduced to alcohols using sodium borohydride, \( \mathrm{NaBH_4} \), or lithium aluminium hydride, \( \mathrm{LiAlH_4} \).
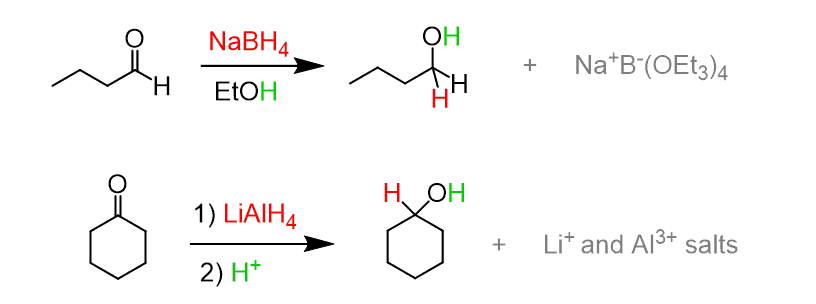
Products:
- Aldehydes → primary alcohols
- Ketones → secondary alcohols
General equations:
\( \mathrm{RCHO \rightarrow RCH_2OH} \)
\( \mathrm{RCOR’ \rightarrow RCHOHR’} \)
(b) Formation of Hydroxynitriles
Aldehydes and ketones react with hydrogen cyanide, using potassium cyanide as a catalyst, to form hydroxynitriles.
Conditions: hydrogen cyanide, catalytic \( \mathrm{KCN} \), gentle heat.
General equations:
\( \mathrm{RCHO + HCN \rightarrow RCH(OH)CN} \)
\( \mathrm{RCOR’ + HCN \rightarrow RC(OH)(CN)R’} \)
This reaction is a nucleophilic addition. The cyanide ion acts as the nucleophile.
Examples
Ethanal forms 2-hydroxypropanenitrile:
\( \mathrm{CH_3CHO + HCN \rightarrow CH_3CH(OH)CN} \)
Propanone forms 2-hydroxy-2-methylpropanenitrile:
\( \mathrm{CH_3COCH_3 + HCN \rightarrow (CH_3)_2C(OH)CN} \)
Example
State the product formed when propanone is reduced using sodium borohydride.
▶️ Answer / Explanation
Propanone is a ketone.
Reduction of a ketone produces a secondary alcohol.
The product is propan-2-ol.
Example
Explain why potassium cyanide is required as a catalyst in the reaction between ethanal and hydrogen cyanide.
▶️ Answer / Explanation
Potassium cyanide provides cyanide ions.
The cyanide ion acts as a nucleophile and attacks the carbonyl carbon.
This initiates nucleophilic addition, allowing hydroxynitrile formation.
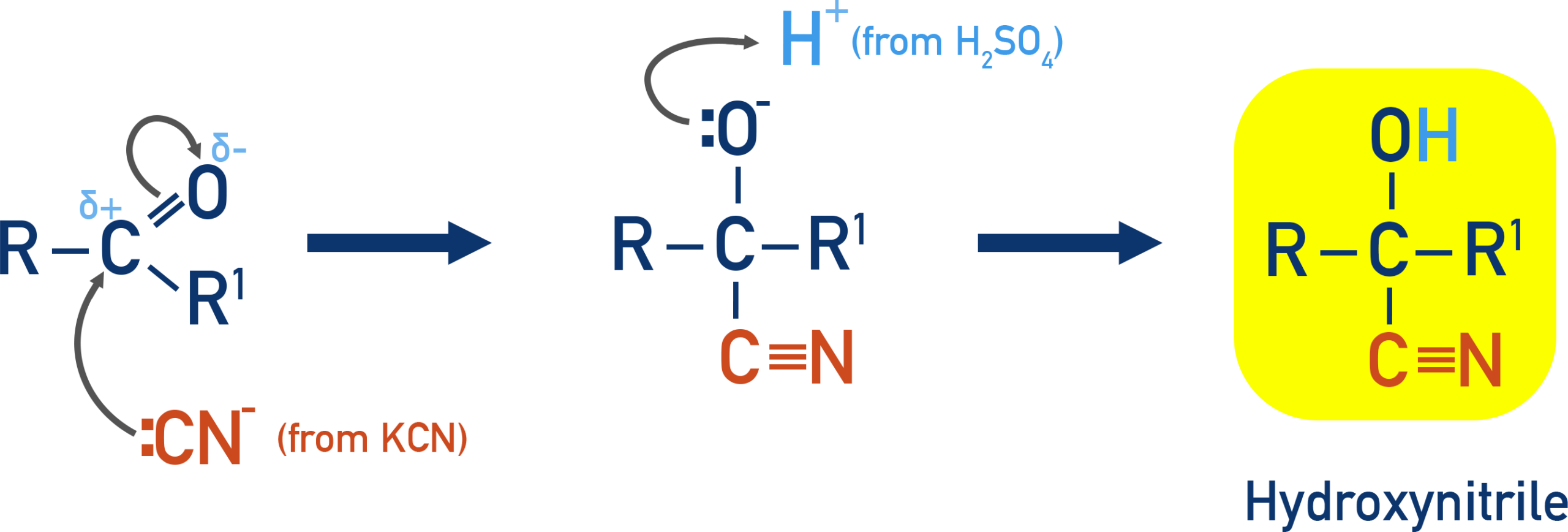
Mechanism of Nucleophilic Addition: Hydrogen Cyanide
Aldehydes and ketones undergo nucleophilic addition reactions because the carbonyl group contains a polar \( \mathrm{C=O} \) bond. This mechanism explains the formation of hydroxynitriles.

Polarity of the Carbonyl Group
In the carbonyl group, oxygen is more electronegative than carbon.
This makes the carbon atom electron deficient and susceptible to attack by a nucleophile.
Overall Reaction
Aldehyde:
\( \mathrm{RCHO + HCN \rightarrow RCH(OH)CN} \)
Ketone:
\( \mathrm{RCOR’ + HCN \rightarrow RC(OH)(CN)R’} \)
Step 1: Nucleophilic Attack
The cyanide ion, \( \mathrm{CN^-} \), acts as a nucleophile.
It attacks the electron-deficient carbonyl carbon, causing the \( \mathrm{C=O} \) double bond to break.
This forms a tetrahedral intermediate containing an alkoxide ion:
\( \mathrm{RCH(O^- )CN} \) (aldehyde example)
Step 2: Protonation
The negatively charged oxygen atom is protonated by hydrogen cyanide, \( \mathrm{HCN} \).
This forms the hydroxyl group and regenerates the cyanide ion:
\( \mathrm{RCH(O^- )CN + HCN \rightarrow RCH(OH)CN + CN^-} \)
Because the cyanide ion is regenerated, potassium cyanide acts as a catalyst.
Key Features of the Mechanism
- The reaction is a nucleophilic addition
- The cyanide ion is the nucleophile
- The carbonyl carbon is the electrophile
- The reaction occurs in two steps
Example
Explain why aldehydes are susceptible to nucleophilic attack by cyanide ions.
▶️ Answer / Explanation
The carbonyl group in an aldehyde is polar.
The carbonyl carbon is electron deficient.
This allows nucleophiles such as \( \mathrm{CN^-} \) to attack the carbonyl carbon.
Example
Explain the role of hydrogen cyanide in the second step of the nucleophilic addition mechanism.
▶️ Answer / Explanation
Hydrogen cyanide donates a proton to the alkoxide ion.
This converts the alkoxide ion into a hydroxyl group.
The cyanide ion is regenerated, allowing it to act as a catalyst.
Test for Carbonyl Compounds: 2,4-Dinitrophenylhydrazine (2,4-DNPH)
Aldehydes and ketones can be identified using 2,4-dinitrophenylhydrazine, commonly known as 2,4-DNPH reagent. This is a qualitative test for the carbonyl group, \( \mathrm{C=O} \).
Reagent
2,4-DNPH is used in acidic solution, usually dissolved in methanol and sulfuric acid.
Observation
A yellow or orange precipitate forms if a carbonyl compound (aldehyde or ketone) is present.
Reaction
The carbonyl compound reacts with 2,4-DNPH to form a 2,4-dinitrophenylhydrazone.
General equation:
\( \mathrm{R_2C=O + H_2NNHC_6H_3(NO_2)_2 \rightarrow R_2C=NNHC_6H_3(NO_2)_2 + H_2O} \)
Both aldehydes and ketones give a positive result. The test does not distinguish between them.
Key Points for Examinations
- Positive result: yellow/orange precipitate
- Indicates the presence of a carbonyl group
- Works for aldehydes and ketones only
- No reaction with alcohols or carboxylic acids
Example
A compound produces an orange precipitate when warmed with 2,4-DNPH reagent. What functional group is present?
▶️ Answer / Explanation
The orange precipitate indicates a positive 2,4-DNPH test.
This shows the presence of a carbonyl group.
The compound is therefore an aldehyde or a ketone.
Example
A compound gives a positive result with 2,4-DNPH but does not react with Tollens’ reagent. Deduce the type of carbonyl compound present.
▶️ Answer / Explanation
2,4-DNPH confirms the presence of a carbonyl group.
A lack of reaction with Tollens’ reagent means it is not an aldehyde.
The compound must therefore be a ketone.
Distinguishing Aldehydes and Ketones
Aldehydes and ketones both contain the carbonyl group, but they can be distinguished using simple chemical tests based on their ease of oxidation.
Key Principle
Aldehydes are readily oxidised, whereas ketones are resistant to oxidation under mild conditions.
(a) Tollens’ Reagent
Tollens’ reagent contains silver(I) ions in ammonia solution. It is used to test for aldehydes.
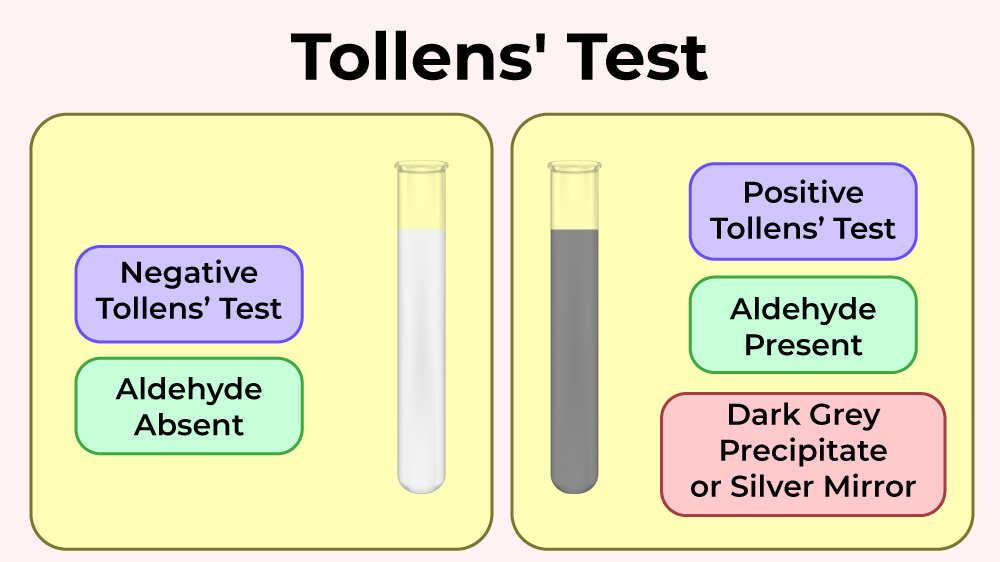
Positive result: formation of a silver mirror or grey precipitate of silver.
Aldehydes are oxidised to carboxylate ions:
\( \mathrm{RCHO + 2Ag^+ \rightarrow RCOO^- + 2Ag} \)
Ketones give no reaction.
(b) Fehling’s Reagent
Fehling’s reagent contains Cu^{2+} ions in alkaline solution.

Positive result: blue solution changes to a brick-red precipitate of copper(I) oxide.
Oxidation of an aldehyde:
\( \mathrm{RCHO + 2Cu^{2+} \rightarrow RCOO^- + Cu_2O} \)
Ketones give no reaction with Fehling’s reagent.
(c) Ease of Oxidation
Aldehydes are oxidised under mild conditions, while ketones are not oxidised unless harsh conditions are used.
This difference allows aldehydes and ketones to be distinguished experimentally.
Summary of Results
- Aldehyde → positive Tollens’ and Fehling’s tests
- Ketone → no reaction with either reagent
Example
An unknown carbonyl compound gives a silver mirror with Tollens’ reagent. Deduce the nature of the compound.
▶️ Answer / Explanation
A silver mirror indicates oxidation by Tollens’ reagent.
Only aldehydes are readily oxidised.
The compound is therefore an aldehyde.
Example
An unknown compound gives a positive 2,4-DNPH test but shows no reaction with Fehling’s solution or Tollens’ reagent. Deduce the nature of the carbonyl compound and explain your reasoning.
▶️ Answer / Explanation
The positive 2,4-DNPH test confirms the presence of a carbonyl group.
No reaction with Tollens’ or Fehling’s reagent shows it is not oxidised.
Ketones are resistant to oxidation under these conditions.
The compound is therefore a ketone.
Test for the CH3CO– Group: Iodoform Reaction
Aldehydes and ketones containing the CH3CO– group can be identified using the iodoform test. This is a characteristic reaction of methyl carbonyl compounds.

Reagent
The reagent used is alkaline iodine, \( \mathrm{I_2(aq)} \) with sodium hydroxide.
Observation
A yellow precipitate of tri-iodomethane, \( \mathrm{CHI_3} \), is formed.
Meaning of a Positive Test
A positive iodoform test indicates the presence of a CH3CO– group in an aldehyde or ketone, with the general structure \( \mathrm{CH_3CO{-}R} \).
Reaction
The reaction produces tri-iodomethane and a carboxylate ion, \( \mathrm{RCO_2^-} \).
General equation:
\( \mathrm{CH_3CO{-}R + 3I_2 + 4OH^- \rightarrow CHI_3 + RCO_2^- + 3I^- + 3H_2O} \)
Only compounds containing the CH3CO– group give this reaction. Other aldehydes and ketones give no precipitate.
Examples That Give a Positive Test
- Ethanal, \( \mathrm{CH_3CHO} \)
- Propanone, \( \mathrm{CH_3COCH_3} \)
Example
A carbonyl compound gives a yellow precipitate when warmed with alkaline iodine. What structural feature must be present?
▶️ Answer / Explanation
The yellow precipitate is tri-iodomethane.
This indicates a positive iodoform test.
The compound must contain a CH3CO– group.
Example
A compound gives a positive 2,4-DNPH test and a yellow precipitate with alkaline iodine. Deduce the type of carbonyl compound present.
▶️ Answer / Explanation
The 2,4-DNPH test confirms the presence of a carbonyl group.
The iodoform test confirms the presence of a CH3CO– group.
The compound must therefore be a methyl ketone or ethanal.
