CIE AS/A Level Chemistry 18.1 Carboxylic acids Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 18.1 Carboxylic acids Study Notes – New Syllabus
CIE AS/A Level Chemistry 18.1 Carboxylic acids Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions by which carboxylic acids can be produced:
(a) oxidation of primary alcohols and aldehydes with acidified K₂Cr₂O₇ or acidified KMnO₄ and refluxing
(b) hydrolysis of nitriles with dilute acid or dilute alkali followed by acidification
(c) hydrolysis of esters with dilute acid or dilute alkali and heat followed by acidificationdescribe:
(a) the redox reaction with reactive metals to produce a salt and H₂(g)
(b) the neutralisation reaction with alkalis to produce a salt and H₂O(l)
(c) the acid–base reaction with carbonates to produce a salt and H₂O(l) and CO₂(g)
(d) esterification with alcohols with concentrated H₂SO₄ as catalyst
(e) reduction by LiAlH₄ to form a primary alcohol
Preparation of Carboxylic Acids
Carboxylic acids can be prepared by several important reactions at A level. These reactions usually involve oxidation or hydrolysis, followed by appropriate conditions.
(a) Oxidation of Primary Alcohols and Aldehydes
Primary alcohols and aldehydes can be oxidised to carboxylic acids using acidified potassium dichromate(VI) or acidified potassium manganate(VII), with refluxing.
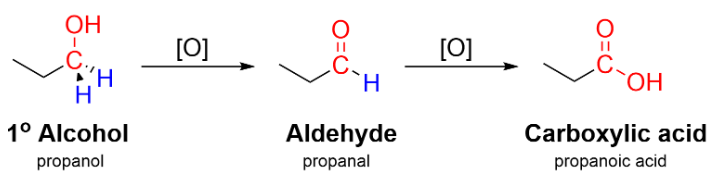
- Primary alcohol → aldehyde → carboxylic acid
- Aldehydes oxidise directly to carboxylic acids
General equation: \( \mathrm{RCH_2OH \rightarrow RCOOH} \)
(b) Hydrolysis of Nitriles
Nitriles can be hydrolysed to carboxylic acids by heating with dilute acid or dilute alkali. If alkali is used, acidification is required to form the carboxylic acid.

General equation: \( \mathrm{RCN \rightarrow RCOOH} \)
(c) Hydrolysis of Esters
Esters can be hydrolysed using dilute acid or dilute alkali and heat. Acidification is required if alkaline hydrolysis is used.
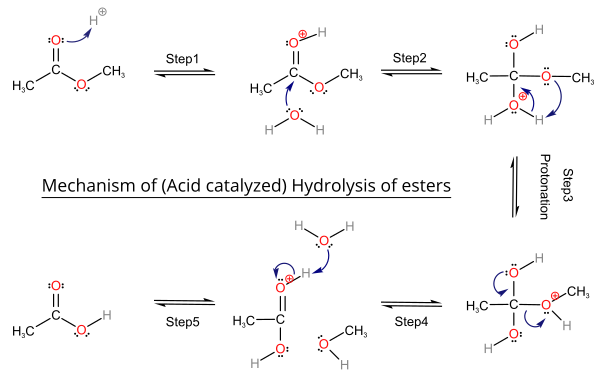
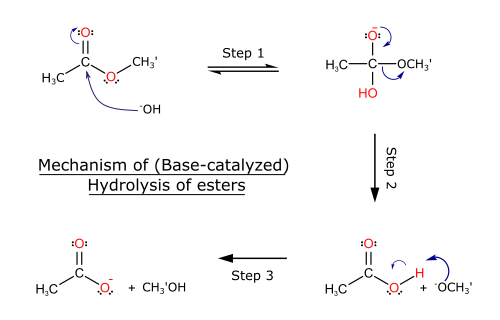
General equation: \( \mathrm{RCOOR’ \rightarrow RCOOH} \)
Example
Describe how propanoic acid can be prepared from propan-1-ol.
▶️ Answer / Explanation
Propan-1-ol is oxidised using acidified potassium dichromate(VI).
The reaction is carried out under reflux.
The primary alcohol is oxidised first to an aldehyde and then to propanoic acid.
Example
Explain why acidification is required after the alkaline hydrolysis of a nitrile to produce a carboxylic acid.
▶️ Answer / Explanation
Alkaline hydrolysis of a nitrile initially forms a carboxylate salt.
The carboxylate ion does not contain the –COOH functional group.
Acidification protonates the carboxylate ion, producing the carboxylic acid.
Reactions of Carboxylic Acids
Carboxylic acids show typical acidic behaviour and also undergo reactions involving their –COOH functional group. The key reactions at A level are outlined below.
(a) Reaction with Reactive Metals
Carboxylic acids react with reactive metals such as magnesium or sodium in a redox reaction to form a metal carboxylate salt and hydrogen gas.
![]()
General equation: \( \mathrm{2RCOOH + Mg \rightarrow (RCOO)_2Mg + H_2} \)
(b) Neutralisation with Alkalis
Carboxylic acids react with alkalis such as sodium hydroxide in a neutralisation reaction to form a salt and water.

General equation: \( \mathrm{RCOOH + NaOH \rightarrow RCOONa + H_2O} \)
(c) Reaction with Carbonates
Carboxylic acids react with carbonates or hydrogencarbonates in an acid–base reaction to produce a salt, water and carbon dioxide gas.
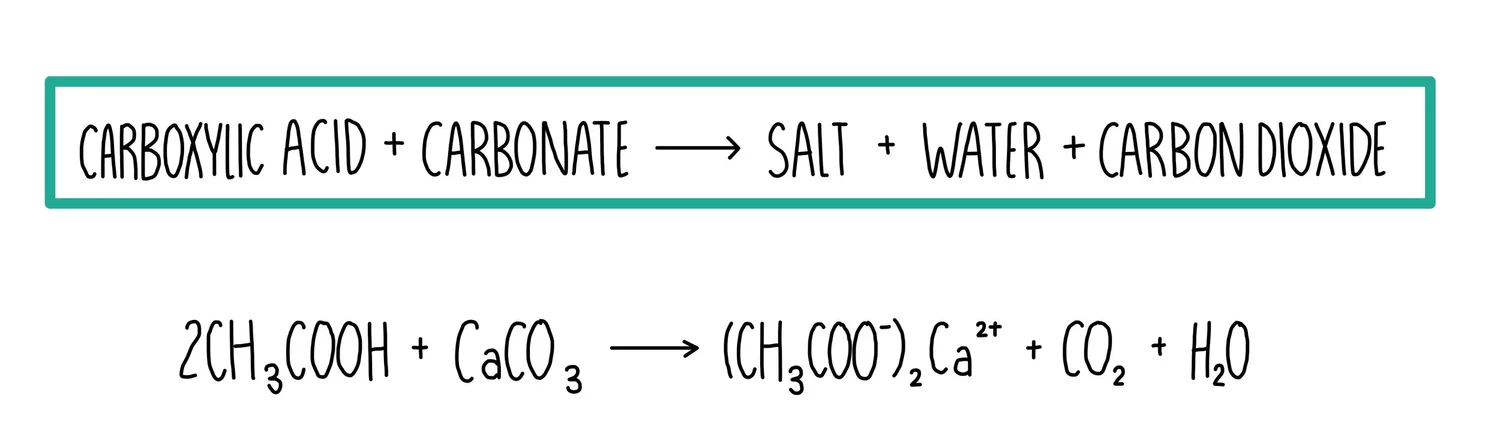
General equation: \( \mathrm{2RCOOH + Na_2CO_3 \rightarrow 2RCOONa + H_2O + CO_2} \)
(d) Esterification with Alcohols
Carboxylic acids react with alcohols in the presence of concentrated sulfuric acid, which acts as a catalyst, to form an ester and water.

General equation: \( \mathrm{RCOOH + R’OH \rightleftharpoons RCOOR’ + H_2O} \)
(e) Reduction with Lithium Aluminium Hydride
Carboxylic acids can be reduced by lithium aluminium hydride, \( \mathrm{LiAlH_4} \), to form primary alcohols.
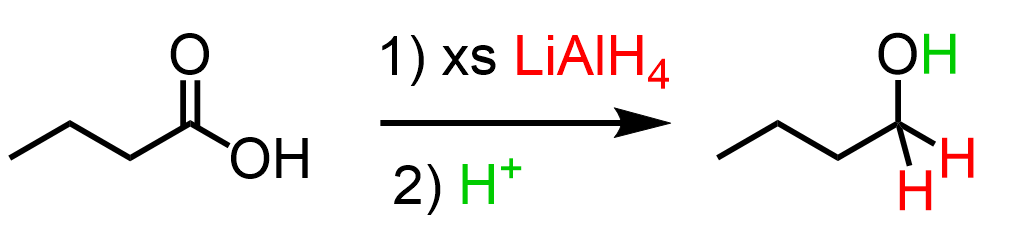
General equation: \( \mathrm{RCOOH \rightarrow RCH_2OH} \)
Example
Describe the reaction between ethanoic acid and sodium carbonate.
▶️ Answer / Explanation
Ethanoic acid reacts with sodium carbonate in an acid–base reaction.
A salt, water and carbon dioxide are produced.
The salt formed is sodium ethanoate.
Example
Explain why concentrated sulfuric acid is used in the esterification of a carboxylic acid with an alcohol.
▶️ Answer / Explanation
Concentrated sulfuric acid acts as a catalyst, increasing the rate of reaction.
It also acts as a dehydrating agent, removing water.
Removing water shifts the equilibrium towards ester formation.
