CIE AS/A Level Chemistry 21.1 Organic synthesis Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 21.1 Organic synthesis Study Notes – New Syllabus
CIE AS/A Level Chemistry 21.1 Organic synthesis Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
for an organic molecule containing several functional groups:
(a) identify organic functional groups using the reactions in the syllabus
(b) predict properties and reactionsdevise multi-step synthetic routes for preparing organic molecules using the reactions in the syllabus
analyse a given synthetic route in terms of type of reaction and reagents used for each step of it, and
possible by-products
Organic Molecules with Multiple Functional Groups
Some organic molecules contain more than one functional group. At A level, you must be able to identify the functional groups present using reactions in the syllabus and then predict the properties and reactions of the molecule.
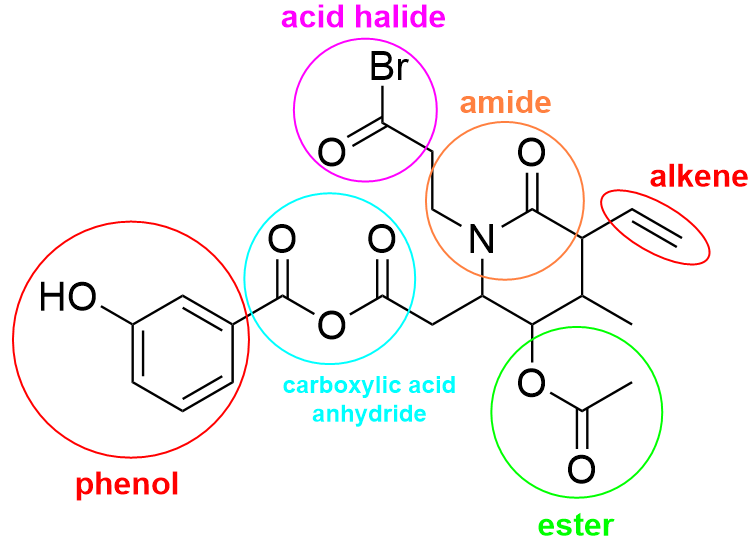
(a) Identifying Functional Groups
Functional groups can be identified by their characteristic reactions. Each functional group reacts independently, even when more than one is present in the same molecule.
- –COOH → acidic behaviour (reaction with carbonates)
- –OH → oxidation or esterification
- C=C → addition reactions
- –NH₂ → basic behaviour (reaction with acids)
- –CN → hydrolysis to carboxylic acids
The presence of one functional group does not prevent another functional group from reacting.
(b) Predicting Properties and Reactions
Once the functional groups are identified, the physical and chemical properties of the molecule can be predicted.
- Acidic or basic nature
- Solubility in water
- Ability to undergo oxidation, reduction, substitution or addition reactions
Predictions should be based on known reactions from the syllabus.
Example
A compound contains both an –OH group and a –COOH group. Identify one reaction that could be used to show the presence of the carboxylic acid group.
▶️ Answer / Explanation
The compound will react with a carbonate.
Effervescence will be observed due to the release of carbon dioxide.
This confirms the presence of a carboxylic acid group.
Example
A molecule contains both a C=C double bond and a carboxylic acid group. Predict two different reactions this molecule could undergo and justify your answer.
▶️ Answer / Explanation
The C=C double bond can undergo addition reactions such as reaction with bromine.
The carboxylic acid group can react with alkalis or carbonates, showing acidic behaviour.
Each functional group reacts independently according to its characteristic chemistry.
Devising Multi-Step Synthetic Routes
General Strategy
- Identify the functional group(s) in the starting molecule
- Identify the functional group(s) required in the product
- Decide which functional group transformations are needed
- Use only known syllabus reactions
- State reagents and conditions for every step
Each step should involve a single clear reaction. Routes are often tested using alcohols, halogenoalkanes, nitriles, carboxylic acids, esters and alkenes.
Common Syllabus Transformations
- Halogenoalkane → alcohol (aqueous hydroxide)
- Halogenoalkane → nitrile (ethanolic KCN, heat)
- Nitrile → carboxylic acid (hydrolysis)
- Alcohol → carboxylic acid (oxidation)
- Carboxylic acid → ester (esterification)
- Ester → alcohol / acid (hydrolysis)
Carbon-chain length changes are often achieved using nitriles.
Example
Devise a two-step synthesis to prepare propanoic acid from bromoethane.
▶️ Answer / Explanation
Step 1: React bromoethane with potassium cyanide in ethanol and heat to form propanenitrile.
Step 2: Hydrolyse propanenitrile using dilute acid or dilute alkali followed by acidification to form propanoic acid.
Example
Devise a three-step synthesis to prepare ethyl propanoate from ethanol.
▶️ Answer / Explanation
Step 1: Oxidise ethanol using acidified potassium dichromate(VI) under reflux to form ethanoic acid.
Step 2: React ethanoic acid with ethanol in the presence of concentrated sulfuric acid and heat under reflux to form ethyl ethanoate.
Step 3: Extend the carbon chain by forming a nitrile intermediate from a suitable halogenoalkane, followed by hydrolysis to propanoic acid, and then esterification with ethanol to form ethyl propanoate.
All steps use reactions from the syllabus with stated reagents and conditions.
Analysing Multi-Step Synthetic Routes
Given a synthetic route and asked to analyse each step in terms of the type of reaction, the reagents and conditions used, and any by-products formed.
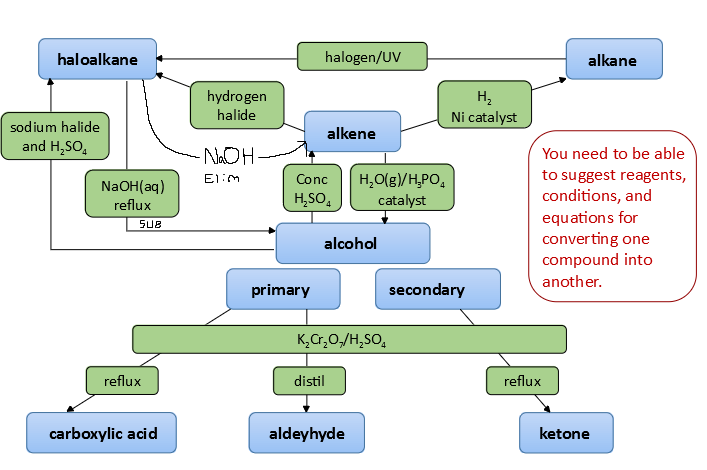
How to Analyse a Synthetic Route
- Identify the functional group change in each step
- State the type of reaction (e.g. oxidation, substitution, hydrolysis)
- Give the correct reagents and conditions
- Identify any small molecules or ions formed as by-products
Each step should be analysed independently. Marks are awarded for clear chemical reasoning, not simply naming the products.
Common Reaction Types and By-products
- Halogenoalkane → nitrile: nucleophilic substitution; by-product = halide ion
- Nitrile → carboxylic acid: hydrolysis; by-products = ammonia or ammonium ions
- Carboxylic acid + alcohol → ester: condensation; by-product = water
- Alcohol → carboxylic acid: oxidation; no organic by-product
Example
Bromoethane is converted into propanoic acid via an intermediate nitrile. Analyse the two steps in this synthesis.
▶️ Answer / Explanation
Step 1: Bromoethane reacts with potassium cyanide in ethanol and heat.
This is a nucleophilic substitution reaction forming propanenitrile.
The by-product is bromide ions.
Step 2: Propanenitrile is hydrolysed using dilute acid or dilute alkali followed by acidification.
This is a hydrolysis reaction producing propanoic acid.
Ammonia or ammonium ions are formed as by-products.
Example
A synthetic route converts a halogenoalkane into an ester via three steps. Analyse each step, stating the type of reaction, reagents used, and any by-products formed.
▶️ Answer / Explanation
Step 1: The halogenoalkane reacts with potassium cyanide in ethanol and heat.
This is nucleophilic substitution forming a nitrile, with halide ions as by-products.
Step 2: The nitrile is hydrolysed with dilute acid or dilute alkali followed by acidification.
This is hydrolysis forming a carboxylic acid, with ammonia or ammonium ions as by-products.
Step 3: The carboxylic acid reacts with an alcohol in the presence of concentrated sulfuric acid and heat.
This is esterification (a condensation reaction), producing an ester and water as a by-product.
