CIE AS/A Level Chemistry 24.1 Electrolysis Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 24.1 Electrolysis Study Notes – New Syllabus
CIE AS/A Level Chemistry 24.1 Electrolysis Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
predict the identities of substances liberated during electrolysis from the state of electrolyte (molten or aqueous), position in the redox series (electrode potential) and concentration
state and apply the relationship F = Le between the Faraday constant, F, the Avogadro constant, L, and the charge on the electron, e
calculate:
(a) the quantity of charge passed during electrolysis, using Q = It
(b) the mass and/or volume of substance liberated during electrolysisdescribe the determination of a value of the Avogadro constant by an electrolytic method
Predicting the Products of Electrolysis
The substances produced during electrolysis depend on:
- Whether the electrolyte is molten or aqueous
- The position of ions in the redox series (electrode potentials)
- The concentration of ions present
(a) Effect of the State of the Electrolyte
Molten electrolytes:

Only ions from the ionic compound are present.
- Metal cations are reduced to metals at the cathode
- Non-metal anions are oxidised at the anode
Aqueous electrolytes:
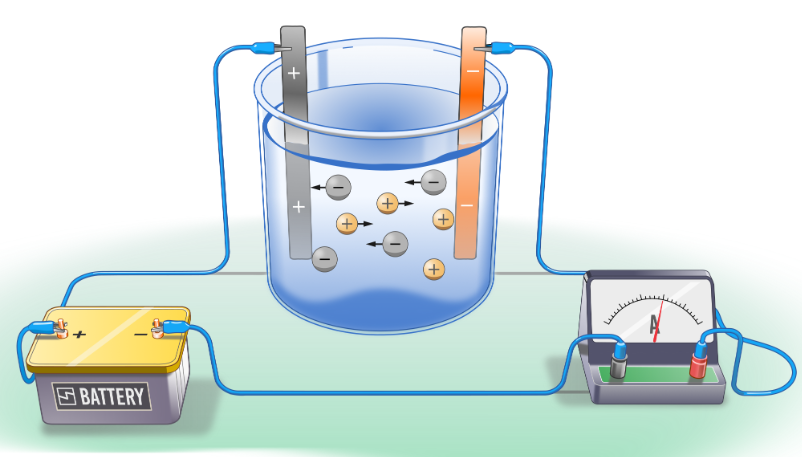
Water provides additional ions, \( \mathrm{H^+} \) and \( \mathrm{OH^-} \), which may be discharged instead.
(b) Effect of the Redox Series (Electrode Potentials)
At the cathode, the species with the most positive electrode potential is preferentially reduced.

In aqueous solutions:
- Metals more reactive than hydrogen are not discharged
- Hydrogen gas is produced instead
- Less reactive metals (e.g. Cu²⁺, Ag⁺) are discharged as metals
At the anode, the species that is most easily oxidised is discharged.
(c) Effect of Concentration
In aqueous solutions containing halide ions:
- Concentrated halide solutions: halogen gas is produced
- Dilute solutions: oxygen is produced instead
This is because higher concentration increases the likelihood of halide ion discharge.
Example
Predict the products at each electrode during the electrolysis of molten sodium chloride.
▶️ Answer / Explanation
Only \( \mathrm{Na^+} \) and \( \mathrm{Cl^-} \) ions are present.
At the cathode, sodium ions gain electrons to form sodium metal.
At the anode, chloride ions lose electrons to form chlorine gas.
Example
Predict the products at each electrode during the electrolysis of aqueous sodium chloride using inert electrodes.
▶️ Answer / Explanation
At the cathode, hydrogen is produced because sodium is more reactive than hydrogen.
At the anode, chlorine gas is produced if the solution is concentrated.
In dilute solution, oxygen would be produced instead.
The products depend on both reactivity and concentration.
Faraday Constant and Charge of the Electron
The Faraday constant links charge measured on a macroscopic scale to the charge of individual electrons using Avogadro’s constant.
Relationship between F, L and e
The relationship is:
\( \mathrm{F = L e} \)
Where:
- \( \mathrm{F} \) is the Faraday constant (approximately \( \mathrm{9.65 \times 10^4\ C\,mol^{-1}} \))
- \( \mathrm{L} \) is the Avogadro constant (\( \mathrm{6.02 \times 10^{23}\ mol^{-1}} \))
- \( \mathrm{e} \) is the charge on one electron (\( \mathrm{1.60 \times 10^{-19}\ C} \))
The Faraday constant represents the charge carried by one mole of electrons.
Applying the Relationship
The equation can be rearranged to find any one quantity:
\( \mathrm{e = \dfrac{F}{L}} \)
\( \mathrm{L = \dfrac{F}{e}} \)
Example
Use the relationship \( \mathrm{F = Le} \) to calculate the charge on one electron.
\( \mathrm{F = 9.65 \times 10^4\ C\,mol^{-1}} \)
\( \mathrm{L = 6.02 \times 10^{23}\ mol^{-1}} \)
▶️ Answer / Explanation
Rearrange the equation:
\( \mathrm{e = \dfrac{F}{L}} \)
\( \mathrm{e = \dfrac{9.65 \times 10^4}{6.02 \times 10^{23}}} \)
\( \mathrm{e = 1.60 \times 10^{-19}\ C} \)
Example
Calculate the total charge carried by electrons when 0.250 mol of electrons pass through a circuit.
▶️ Answer / Explanation
One mole of electrons carries a charge equal to the Faraday constant.
\( \mathrm{Charge = nF} \)
\( \mathrm{Charge = 0.250 \times 9.65 \times 10^4} \)
\( \mathrm{Charge = 2.41 \times 10^4\ C} \)
Calculations in Electrolysis
During electrolysis, electrical energy is used to drive chemical change. Calculations involve finding the quantity of charge passed and relating this to the amount of substance produced.
(a) Quantity of Charge, \( \mathrm{Q} \)
The quantity of charge passed during electrolysis is calculated using:
\( \mathrm{Q = It} \)
Where:
- \( \mathrm{Q} \) is the charge in coulombs, \( \mathrm{C} \)
- \( \mathrm{I} \) is the current in amperes, \( \mathrm{A} \)
- \( \mathrm{t} \) is the time in seconds, \( \mathrm{s} \)
(b) Mass or Volume of Substance Liberated
Once the charge is known, the number of moles of electrons transferred is found using:
\( \mathrm{n(e^-) = \dfrac{Q}{F}} \)
where \( \mathrm{F = 9.65 \times 10^4\ C\,mol^{-1}} \).
The moles of product formed are then found using the half-equation stoichiometry.
Mass is calculated using:
\( \mathrm{m = nM} \)
For gases, volume can be calculated using the molar gas volume (\( \mathrm{24.0\ dm^3\,mol^{-1}} \) at RTP).
Example
A current of 2.50 A is passed through molten lead(II) bromide for 10.0 minutes. Calculate the mass of lead produced.
▶️ Answer / Explanation
Convert time to seconds:
\( \mathrm{t = 10.0 \times 60 = 600\ s} \)
Calculate charge:
\( \mathrm{Q = It = 2.50 \times 600 = 1500\ C} \)
Calculate moles of electrons:
\( \mathrm{n(e^-) = \dfrac{1500}{9.65 \times 10^4} = 0.0155\ mol} \)
Half-equation: \( \mathrm{Pb^{2+} + 2e^- \rightarrow Pb} \)
Moles of lead formed:
\( \mathrm{n(Pb) = 0.0155 \div 2 = 0.00775\ mol} \)
Calculate mass:
\( \mathrm{m = 0.00775 \times 207 = 1.60\ g} \)
Example
A current of 3.00 A is passed through dilute sulfuric acid for 25.0 minutes. Calculate the volume of hydrogen gas produced at RTP.
▶️ Answer / Explanation
Convert time to seconds:
\( \mathrm{t = 25.0 \times 60 = 1500\ s} \)
Calculate charge:
\( \mathrm{Q = 3.00 \times 1500 = 4500\ C} \)
Moles of electrons:
\( \mathrm{n(e^-) = \dfrac{4500}{9.65 \times 10^4} = 0.0466\ mol} \)
Half-equation: \( \mathrm{2H^+ + 2e^- \rightarrow H_2} \)
Moles of hydrogen:
\( \mathrm{n(H_2) = 0.0466 \div 2 = 0.0233\ mol} \)
Volume at RTP:
\( \mathrm{V = 0.0233 \times 24.0 = 0.559\ dm^3} \)
Determination of the Avogadro Constant by an Electrolytic Method
The Avogadro constant, \( \mathrm{L} \), can be determined experimentally using electrolysis by linking the charge passed to the amount of substance produced.
Principle of the Method
Electrolysis is used to measure the charge carried by a known number of electrons. This allows the calculation of the charge of one electron, and hence the Avogadro constant.
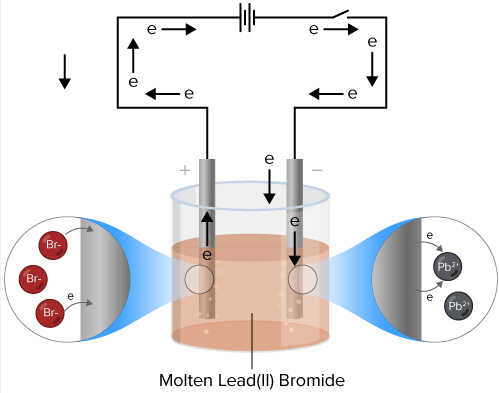
Experimental Method
- Set up an electrolytic cell with inert electrodes
- Electrolyse a suitable electrolyte (e.g. acidified water)
- Measure the current and the time of electrolysis
- Measure the volume or mass of product formed at an electrode
Key Calculations
1. Calculate the charge passed:
\( \mathrm{Q = It} \)
2. Use the half-equation to calculate the moles of electrons transferred.
3. Calculate the charge per mole of electrons (the Faraday constant):
\( \mathrm{F = \dfrac{Q}{n(e^-)}} \)
4. Use the relationship \( \mathrm{F = Le} \) to calculate the Avogadro constant:
\( \mathrm{L = \dfrac{F}{e}} \)
where \( \mathrm{e = 1.60 \times 10^{-19}\ C} \).
Example
During electrolysis, a current of 1.20 A is passed for 20.0 minutes and 0.00750 mol of electrons are transferred. Calculate the Faraday constant.
▶️ Answer / Explanation
Convert time to seconds:
\( \mathrm{t = 20.0 \times 60 = 1200\ s} \)
Calculate charge:
\( \mathrm{Q = It = 1.20 \times 1200 = 1440\ C} \)
Calculate Faraday constant:
\( \mathrm{F = \dfrac{1440}{0.00750} = 1.92 \times 10^5\ C\,mol^{-1}} \)
Example
In an electrolytic experiment, hydrogen gas is collected at the cathode. A current of 2.00 A flows for 15.0 minutes, producing 0.00467 mol of hydrogen. Determine a value for the Avogadro constant.
▶️ Answer / Explanation
Convert time to seconds:
\( \mathrm{t = 15.0 \times 60 = 900\ s} \)
Calculate charge:
\( \mathrm{Q = 2.00 \times 900 = 1800\ C} \)
Half-equation: \( \mathrm{2H^+ + 2e^- \rightarrow H_2} \)
Moles of electrons:
\( \mathrm{n(e^-) = 2 \times 0.00467 = 0.00934\ mol} \)
Calculate Faraday constant:
\( \mathrm{F = \dfrac{1800}{0.00934} = 1.93 \times 10^5\ C\,mol^{-1}} \)
Calculate Avogadro constant:
\( \mathrm{L = \dfrac{1.93 \times 10^5}{1.60 \times 10^{-19}} = 1.21 \times 10^{24}\ mol^{-1}} \)
