CIE AS/A Level Chemistry 27.1 Similarities and trends in the properties of the Group 2 Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 27.1 Similarities and trends in the properties of the Group 2 Study Notes – New Syllabus
CIE AS/A Level Chemistry 27.1 Similarities and trends in the properties of the Group 2 Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
- describe and explain qualitatively the trend in the thermal stability of the nitrates and carbonates including the effect of ionic radius on the polarisation of the large anion
- describe and explain qualitatively the variation in solubility and of enthalpy change of solution, ΔH⦵ sol, of the hydroxides and sulfates in terms of relative magnitudes of the enthalpy change of hydration and the lattice energy
Thermal Stability of Nitrates and Carbonates
The thermal stability of nitrates and carbonates varies across a group in the Periodic Table. This trend can be explained qualitatively using the ideas of ionic radius and polarisation.
Observed Trend
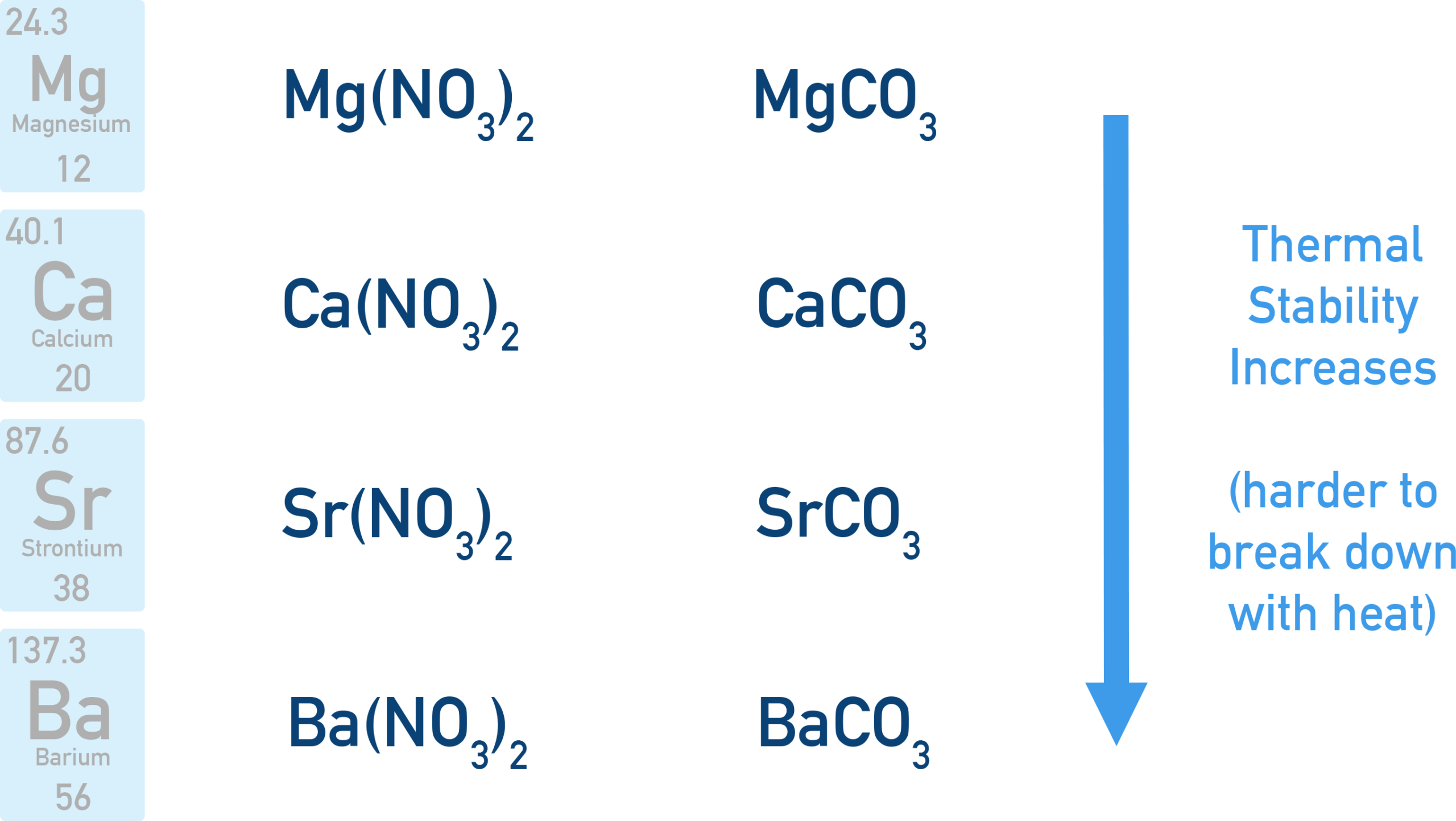
As you move down a group (e.g. Group 2), the thermal stability of both nitrates and carbonates increases.
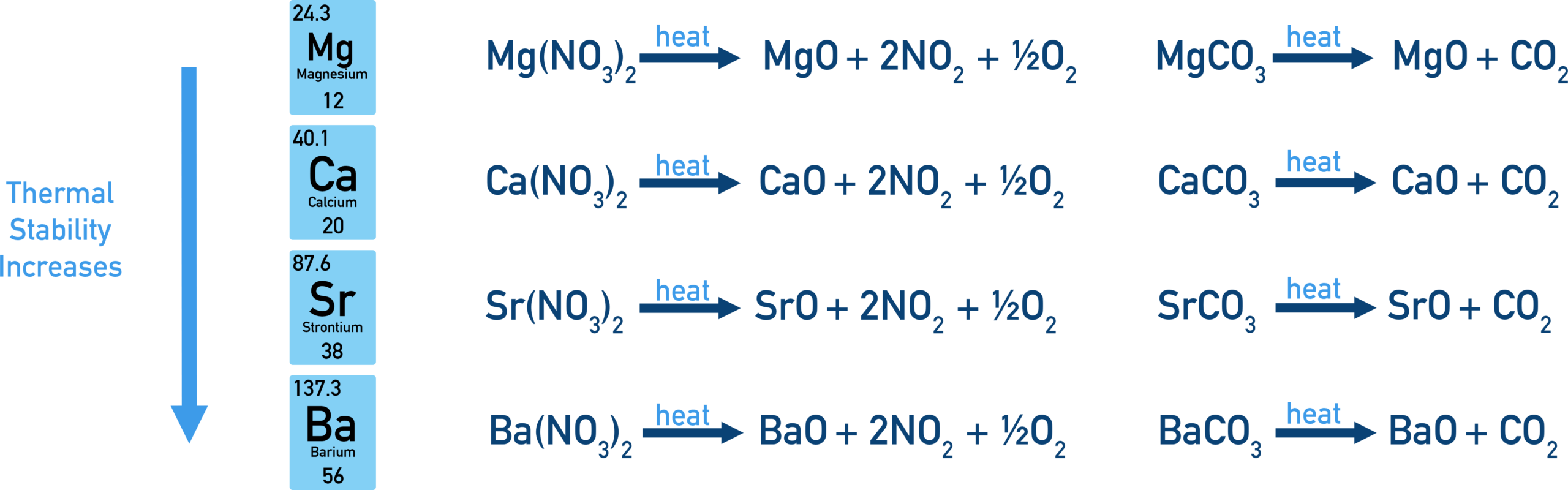
Explanation: Ionic Radius and Polarisation
Both the nitrate ion, \( \mathrm{NO_3^-} \), and the carbonate ion, \( \mathrm{CO_3^{2-}} \), are large, negatively charged anions.
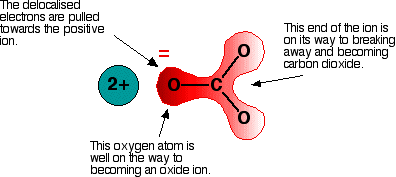
- Small cations have a high charge density
- They strongly polarise the large anion
- This distorts the electron cloud of the anion and weakens bonds within it
At the top of a group, cations have a smaller ionic radius, so they polarise the anion more strongly. This makes the compound less thermally stable and easier to decompose when heated.
 Down the group:
Down the group:
- The ionic radius of the cation increases
- Charge density decreases
- Polarisation of the large anion decreases
- The anion is less distorted and more stable to heat
Therefore, nitrates and carbonates become more thermally stable down the group.
Comparison of Nitrates and Carbonates
Carbonates contain the doubly charged \( \mathrm{CO_3^{2-}} \) ion, which is more easily polarised than the singly charged \( \mathrm{NO_3^-} \) ion.
As a result, carbonates are generally less thermally stable than nitrates for the same metal.
Example
Describe and explain the trend in the thermal stability of Group 2 carbonates.
▶️ Answer / Explanation
The thermal stability of Group 2 carbonates increases down the group.
Down the group, the metal cations have a larger ionic radius and lower charge density.
This reduces polarisation of the large \( \mathrm{CO_3^{2-}} \) ion, so the bonds within the carbonate ion are less weakened.
As a result, the carbonates require higher temperatures to decompose.
Example
Magnesium nitrate decomposes at a much lower temperature than barium nitrate. Explain this difference in terms of polarisation.
▶️ Answer / Explanation
Magnesium ions have a smaller ionic radius and higher charge density than barium ions.
The \( \mathrm{Mg^{2+}} \) ion polarises the large \( \mathrm{NO_3^-} \) ion more strongly, distorting its electron cloud.
This weakens bonds within the nitrate ion, making magnesium nitrate less thermally stable.
Barium ions polarise the nitrate ion less, so barium nitrate is more stable and decomposes at a higher temperature.
Solubility of Hydroxides and Sulfates
The solubility of hydroxides and sulfates varies down a group (commonly Group 2). These trends can be explained qualitatively using the relative magnitudes of the enthalpy change of hydration and the lattice energy.
Enthalpy Change of Solution
The standard enthalpy change of solution, \( \mathrm{\Delta H^\circ_{sol}} \), is the enthalpy change when 1 mole of an ionic solid dissolves in water to form aqueous ions.

It is given by:
\( \mathrm{\Delta H^\circ_{sol} = \Delta H^\circ_{hyd} + \Delta H^\circ_{latt}} \)
- \( \mathrm{\Delta H^\circ_{hyd}} \) is always negative (energy released when ions are hydrated)
- \( \mathrm{\Delta H^\circ_{latt}} \) is positive (energy required to separate the ionic lattice)

Hydroxides: Observed Trend The solubility of Group 2 hydroxides increases down the group. Hydroxides: Explanation Down the group, the metal cation becomes larger.
The decrease in lattice energy is greater than the decrease in hydration enthalpy, so \( \mathrm{\Delta H^\circ_{sol}} \) becomes more negative down the group. As dissolution becomes more exothermic, the hydroxides become more soluble. | Sulfates: Observed Trend The solubility of Group 2 sulfates decreases down the group. Sulfates: Explanation Sulfate ions, \( \mathrm{SO_4^{2-}} \), are large and doubly charged.
Overall, the reduction in hydration enthalpy outweighs the reduction in lattice energy. This makes \( \mathrm{\Delta H^\circ_{sol}} \) less negative or positive. As dissolution becomes less energetically favourable, sulfate solubility decreases. |
Example
Describe and explain the trend in solubility of Group 2 hydroxides down the group.
▶️ Answer / Explanation
The solubility of Group 2 hydroxides increases down the group.
Down the group, the metal ions increase in size, causing the lattice energy to decrease.
Although hydration enthalpy becomes less negative, the lattice energy decreases by a greater amount.
This makes \( \mathrm{\Delta H^\circ_{sol}} \) more negative, so the hydroxides dissolve more readily.
Example
Barium hydroxide is soluble in water, whereas barium sulfate is almost insoluble. Explain this difference in terms of hydration enthalpy and lattice energy.
▶️ Answer / Explanation
In barium hydroxide, the decrease in lattice energy is greater than the decrease in hydration enthalpy.
This results in a negative \( \mathrm{\Delta H^\circ_{sol}} \), making dissolution energetically favourable.
In barium sulfate, the hydration enthalpy becomes much less negative for the large ions.
This outweighs the decrease in lattice energy, giving a positive or near-zero \( \mathrm{\Delta H^\circ_{sol}} \), so barium sulfate is insoluble.
