CIE AS/A Level Chemistry 28.1 General physical and chemical properties of the first row of transition elements Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 28.1 General physical and chemical properties of the first row of transition elements Study Notes – New Syllabus
CIE AS/A Level Chemistry 28.1 General physical and chemical properties of the first row of transition elements Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
define a transition element as a d-block element which forms one or more stable ions with incomplete d orbitals
sketch the shape of a 3dₓᵧ orbital and 3dᶻ² orbital
understand that transition elements have the following properties:
(a) they have variable oxidation states
(b) they behave as catalysts
(c) they form complex ions
(d) they form coloured compoundsexplain why transition elements have variable oxidation states in terms of the similarity in energy of the 3d and the 4s sub-shells
explain why transition elements behave as catalysts in terms of having more than one stable oxidation state, and vacant d orbitals that are energetically accessible and can form dative bonds with ligands
explain why transition elements form complex ions in terms of vacant d orbitals that are energetically accessible
Transition Elements
A transition element is defined based on the electronic structure of its ions.

- A transition element is a d-block element which forms one or more stable ions with an incomplete d subshell.
- When a transition element forms ions, electrons are lost from the 4s orbital before the 3d orbital.
- If the resulting ion has a partially filled d orbital, the element is classified as a transition element.
Important Note
- Not all d-block elements are transition elements
- For example, zinc forms \( \mathrm{Zn^{2+}} \) ions with a full d subshell, so zinc is not a transition element
Example
Explain why iron is a transition element.
▶️ Answer / Explanation
Iron is a d-block element that forms \( \mathrm{Fe^{2+}} \) and \( \mathrm{Fe^{3+}} \) ions.
These ions have incomplete d orbitals, so iron is classified as a transition element.
Example
Copper and zinc are both d-block elements. Explain why copper is a transition element but zinc is not.
▶️ Answer / Explanation
Copper forms \( \mathrm{Cu^{2+}} \) ions with an incomplete d subshell.
Zinc forms only \( \mathrm{Zn^{2+}} \) ions with a full d subshell.
Therefore, copper is a transition element whereas zinc is not.
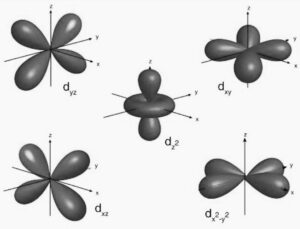
Shapes of d Orbitals
The shapes of d orbitals describe regions of space where there is a high probability of finding an electron. You are expected to recognise and sketch the shapes of individual d orbitals.
The 3dxy Orbital

The 3dxy orbital:
- Has four lobes
- Lies in the xy-plane
- The lobes are oriented between the x- and y-axes, not along them
The 3dz² Orbital

The 3dz² orbital:
- Has two lobes along the z-axis
- Has a ring (torus) of electron density in the xy-plane
- Is symmetrical around the z-axis
Example
State one difference between the shapes of the 3dxy and 3dz² orbitals.
▶️ Answer / Explanation
The 3dxy orbital has four lobes between the x- and y-axes, whereas the 3dz² orbital has two lobes along the z-axis and a ring in the xy-plane.
Example
Explain why the 3dz² orbital does not have the same shape as the other four d orbitals.
▶️ Answer / Explanation
The 3dz² orbital has electron density concentrated along the z-axis and an additional ring in the xy-plane.
The other d orbitals consist of four lobes oriented either between or along axes, giving them a different symmetry.
Properties of Transition Elements
Transition elements show a number of characteristic properties due to their partially filled d orbitals. These properties distinguish them from s-block elements.
(a) Variable Oxidation States
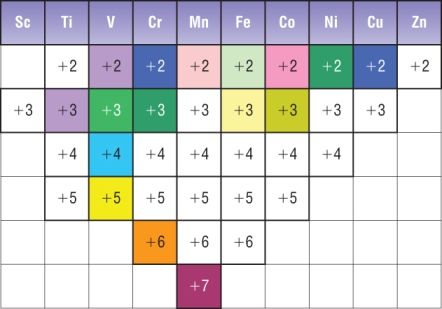
- Transition elements can form ions with different oxidation states.
- This occurs because the 4s and 3d electrons are similar in energy, so different numbers of electrons can be lost.
For example, iron commonly forms \( \mathrm{Fe^{2+}} \) and \( \mathrm{Fe^{3+}} \) ions.
(b) Catalytic Behaviour
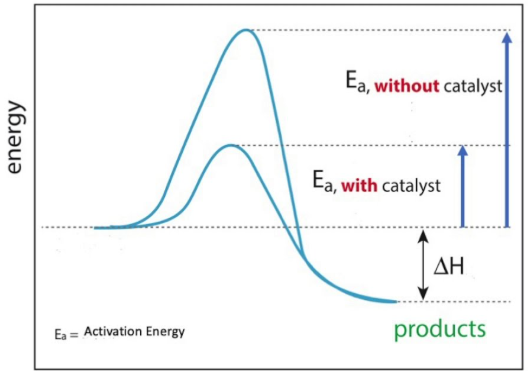
- Transition elements and their compounds often act as catalysts.
- They can provide an alternative reaction pathway by:
- Forming intermediate compounds
- Changing oxidation state during the reaction
- At the end of the reaction, the catalyst is regenerated.
(c) Formation of Complex Ions
- Transition metal ions form complex ions by bonding with ligands.
- Ligands donate lone pairs of electrons into empty d orbitals of the metal ion, forming coordinate (dative) bonds.
| Structure | Central atom / ion | Ligand(s) | Ligand number | Name of complex ion |
|---|---|---|---|---|
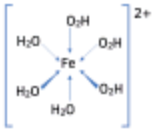 | Iron(II) | Water | 6 | Hexa-aqua-iron(II) ion |
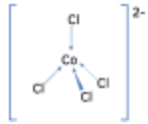 | Cobalt(II) | Chloride | 4 | Tetra-chloro-cobalt(II) ion |
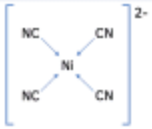 | Nickel(II) | Cyanide | 4 | Tetra-cyano-nickel(II) ion |
 | Silver | Ammonia | 2 | Diamminesilver ion |
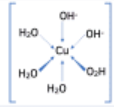 | Copper(II) | Water Hydroxide ions | 2 3 | Di-hydroxy-tetra-aqua-copper(II) ion |
For example: \( \mathrm{[Cu(H_2O)_6]^{2+}} \)
(d) Formation of Coloured Compounds
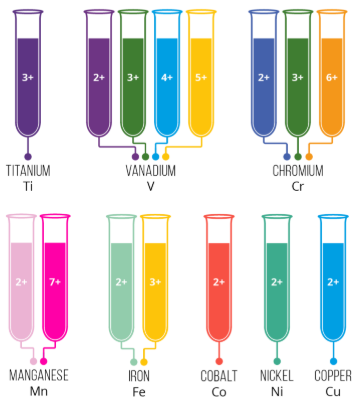
- Many transition metal compounds are coloured.
- This is because electrons in partially filled d orbitals can absorb visible light and move between energy levels.
- The colour observed is the complementary colour of the light absorbed.
Example
State two properties of transition elements and explain one of them.
▶️ Answer / Explanation
Transition elements have variable oxidation states and form coloured compounds.
They have variable oxidation states because both 4s and 3d electrons can be lost.
Example
Explain why transition elements are good catalysts.
▶️ Answer / Explanation
Transition elements can change oxidation state during a reaction.
This allows them to form intermediates and provide an alternative pathway with a lower activation energy.
The transition element is regenerated at the end of the reaction.
Variable Oxidation States of Transition Elements
A key characteristic of transition elements is that they can exist in more than one oxidation state. This behaviour can be explained by considering the relative energies of the 3d and 4s sub-shells.
Energy of 3d and 4s Sub-shells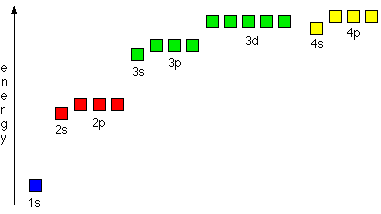
In transition elements, the 3d and 4s sub-shells are very similar in energy.
As a result:
- Electrons can be removed from both the 4s and 3d sub-shells
- Different numbers of electrons can be lost with similar energy requirements
Formation of Different Oxidation States
When a transition element forms ions:
- The 4s electrons are lost first
- Further electrons may then be lost from the 3d sub-shell
Because the energy difference between removing 4s and 3d electrons is small, the element can form ions with different numbers of electrons removed, leading to variable oxidation states.
Example
Iron has the electronic configuration \( \mathrm{[Ar]\,3d^6\,4s^2} \).
Iron can form:
- \( \mathrm{Fe^{2+}} \) by losing two 4s electrons
- \( \mathrm{Fe^{3+}} \) by losing two 4s electrons and one 3d electron
Both ions are stable because the 3d and 4s sub-shells are close in energy.
Example
Explain why chromium shows variable oxidation states.
▶️ Answer / Explanation
In chromium, the 3d and 4s sub-shells are close in energy.
This means electrons can be lost from both sub-shells.
As a result, chromium can lose different numbers of electrons and form ions with different oxidation states.
Example
Explain why transition elements commonly show more variable oxidation states than s-block elements.
▶️ Answer / Explanation
In transition elements, the 3d and 4s sub-shells are similar in energy.
This allows electrons to be removed from both sub-shells with comparable energy.
In s-block elements, only the outer s electrons are available, so they form ions with fixed oxidation states.
Why Transition Elements Behave as Catalysts
Transition elements and their compounds often act as effective catalysts. This behaviour can be explained by their ability to change oxidation state and by the presence of energetically accessible vacant d orbitals.
Role of Variable Oxidation States
Transition elements have more than one stable oxidation state.

This allows them to:
- Gain or lose electrons during a reaction
- Participate in redox cycles
- Be used up in one step and reformed in a later step
As a result, transition elements can provide an alternative reaction pathway with a lower activation energy and are regenerated at the end of the reaction.
Role of Vacant d Orbitals
Transition metal ions have vacant d orbitals that are close in energy to the filled orbitals.

These vacant d orbitals can:
- Accept lone pairs from ligands
- Form dative (coordinate) bonds
- Stabilise intermediate species
The formation of these temporary bonds helps hold reactant molecules close together, weakens existing bonds, and lowers the activation energy.
Thus, transition elements behave as catalysts because:
- They can change oxidation state during the reaction
- They have vacant, accessible d orbitals that form dative bonds with ligands
- They form intermediates and are regenerated at the end of the reaction
Example
Explain why transition metal ions often act as homogeneous catalysts.
▶️ Answer / Explanation
Transition metal ions have more than one stable oxidation state.
This allows them to gain and lose electrons during the reaction, so they are used in one step and reformed in a later step.
They also have vacant d orbitals that can accept lone pairs from ligands, forming temporary dative bonds and stabilising intermediates.
Example
Explain, with reference to electronic structure, why transition metals are more effective catalysts than s-block metals.
▶️ Answer / Explanation
Transition metals have accessible vacant d orbitals, whereas s-block metals do not.
These d orbitals can form dative bonds with ligands, allowing transition metals to form intermediates and stabilise reacting species.
Transition metals also have variable oxidation states, enabling them to take part in redox cycles and be regenerated.
Together, these factors allow transition metals to lower activation energy and act as effective catalysts.
Formation of Complex Ions by Transition Elements
Transition elements readily form complex ions. This behaviour can be explained by the presence of vacant d orbitals that are energetically accessible.
Complex Ions
A complex ion consists of a central metal ion surrounded by ligands, bonded via coordinate (dative) bonds.

Vacant d Orbitals
Transition metal ions have vacant d orbitals that are:
- Low in energy
- Close in energy to occupied orbitals
- Available to accept lone pairs
Because these d orbitals are energetically accessible, they can accept electron pairs donated by ligands such as \( \mathrm{H_2O} \), \( \mathrm{NH_3} \), or \( \mathrm{Cl^-} \).
Formation of Dative Bonds
Ligands act as Lewis bases and donate a lone pair of electrons into a vacant d orbital of the metal ion. This forms a dative (coordinate) bond, where both electrons in the bond come from the ligand.
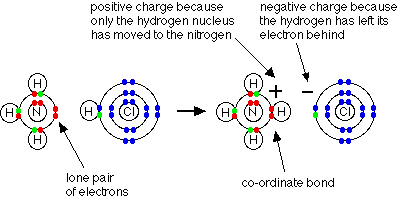
Thus, transition elements form complex ions because:
- They have vacant d orbitals
- These orbitals are energetically accessible
- They can accept lone pairs from ligands to form stable coordinate bonds
Example
Explain why \( \mathrm{Cu^{2+}} \) ions form complex ions in aqueous solution.
▶️ Answer / Explanation
\( \mathrm{Cu^{2+}} \) has vacant d orbitals that are energetically accessible.
Water molecules donate lone pairs into these orbitals, forming dative bonds.
This results in the formation of a complex ion such as \( \mathrm{[Cu(H_2O)_6]^{2+}} \).
Example
Explain why transition elements form complex ions whereas s-block elements do not.
▶️ Answer / Explanation
Transition elements have vacant d orbitals that are low in energy and accessible.
These orbitals can accept lone pairs from ligands to form dative bonds.
S-block elements do not have accessible d orbitals, so they cannot readily form complex ions.
