CIE AS/A Level Chemistry 28.3 Colour of complexes Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 28.3 Colour of complexes Study Notes – New Syllabus
CIE AS/A Level Chemistry 28.3 Colour of complexes Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
define and use the terms degenerate and non-degenerate d orbitals
describe the splitting of degenerate d orbitals into two non-degenerate sets of d orbitals of higher energy, and use of ΔE in:
(a) octahedral complexes, two higher and three lower d orbitals
(b) tetrahedral complexes, three higher and two lower d orbitalsexplain why transition elements form coloured compounds in terms of the frequency of light absorbed as an electron is promoted between two non-degenerate d orbitals
describe, in qualitative terms, the effects of different ligands on ΔE, frequency of light absorbed, and hence the complementary colour that is observed
use the complexes of copper(II) ions and cobalt(II) ions with water and ammonia molecules and hydroxide and chloride ions as examples of ligand exchange affecting the colour observed
Degenerate and Non-degenerate d Orbitals
The terms degenerate and non-degenerate are used to describe whether orbitals have the same energy or different energies. They are particularly important when explaining the behaviour of d orbitals in transition metal complexes.
Degenerate d Orbitals
Degenerate orbitals are orbitals that have the same energy.

- In an isolated transition metal ion or atom (i.e. with no ligands present), all five d orbitals are degenerate.

- These orbitals are:
- 3dxy
- 3dxz
- 3dyz
- 3dx²−y²
- 3dz²
- Because they all have the same energy, electrons distribute evenly among them according to Hund’s rule.
Non-degenerate d Orbitals
Non-degenerate orbitals are orbitals that have different energies.
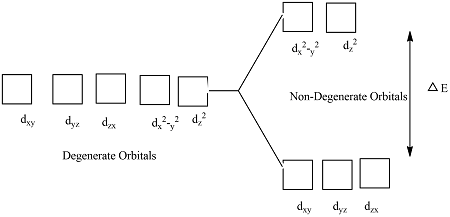
- When ligands bond to a transition metal ion to form a complex, the five d orbitals experience different repulsions from the ligands. This causes the d orbitals to split into different energy levels.
- As a result, the d orbitals are no longer degenerate.
Example: Octahedral Complexes
In an octahedral complex:
- Three d orbitals become lower in energy (still degenerate with each other)
- Two d orbitals become higher in energy (degenerate with each other)
Although the orbitals split into groups, orbitals within each group remain degenerate.
Example
State what is meant by the term degenerate d orbitals.
▶️ Answer / Explanation
Degenerate d orbitals are d orbitals that have the same energy.
Example
Explain why d orbitals are non-degenerate in a transition metal complex.
▶️ Answer / Explanation
When ligands approach the metal ion, their lone pairs repel electrons in the d orbitals.
Different d orbitals experience different amounts of repulsion depending on their orientation.
This causes the d orbitals to split into orbitals of different energies, making them non-degenerate.
Splitting of d Orbitals in Transition Metal Complexes
- In a free transition metal ion, the five d orbitals are degenerate (i.e. they have the same energy).
- When ligands bond to the metal ion to form a complex, the d orbitals split into two non-degenerate energy levels.
- This splitting occurs because ligands repel electrons in the d orbitals to different extents depending on the orientation of the orbital relative to the ligands.
Crystal Field Splitting Energy, \( \mathrm{\Delta E} \)
\( \mathrm{\Delta E} \) is the energy difference between the two sets of non-degenerate d orbitals formed when ligands approach a metal ion.

The size of \( \mathrm{\Delta E} \) depends on:
- The geometry of the complex
- The metal ion
- The nature of the ligands
(a) Octahedral Complexes
In an octahedral complex, six ligands approach the metal ion along the x-, y- and z-axes.
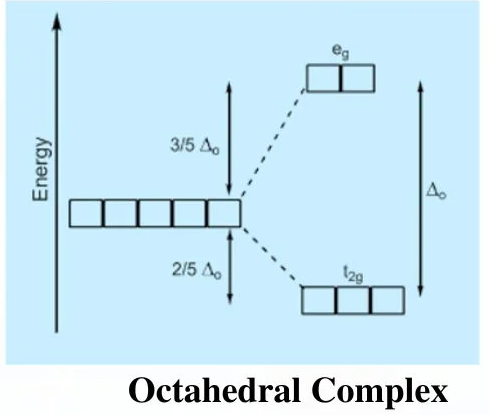
As a result, the five degenerate d orbitals split into:
- Two higher-energy d orbitals (dx²−y² and dz²)
- Three lower-energy d orbitals (dxy, dxz and dyz)
The orbitals pointing directly at the ligands experience greater repulsion and therefore have higher energy.
The energy gap between the two sets is the octahedral splitting energy, \( \mathrm{\Delta E} \).
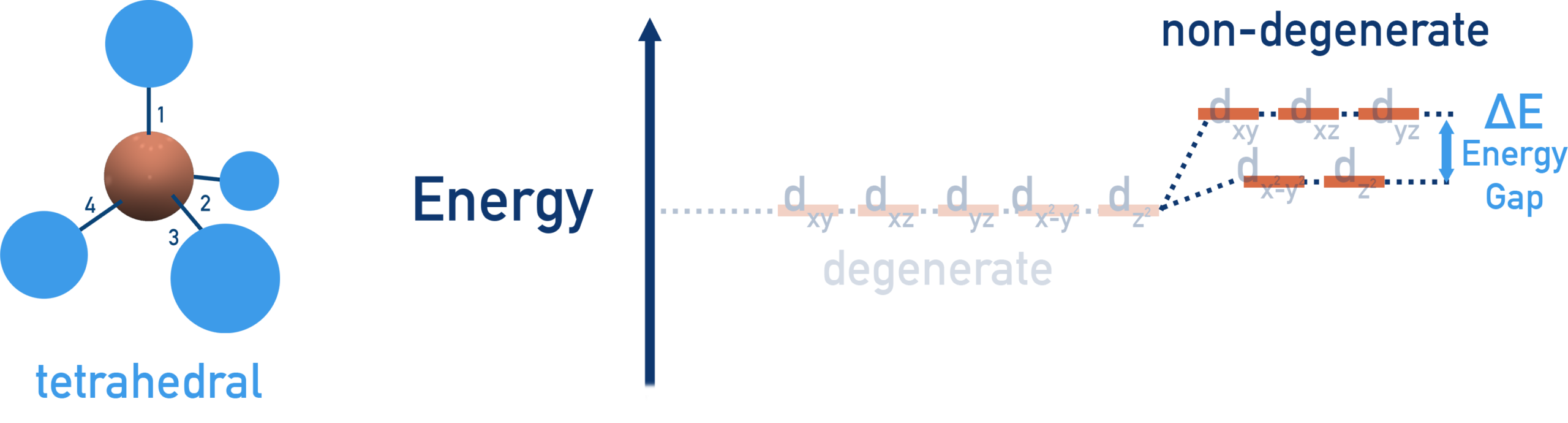
(b) Tetrahedral Complexes
In a tetrahedral complex, four ligands approach the metal ion between the axes rather than directly along them.
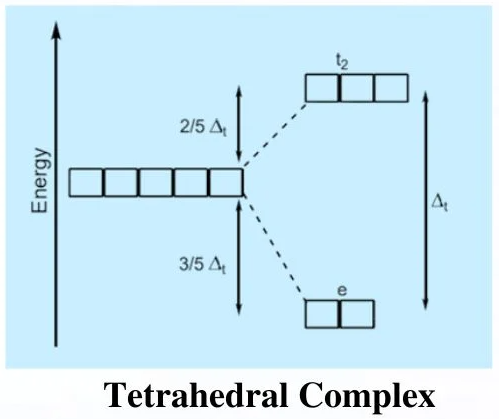
This causes the d orbitals to split into:
- Three higher-energy d orbitals (dxy, dxz and dyz)
- Two lower-energy d orbitals (dx²−y² and dz²)
The pattern of splitting is the opposite of that in octahedral complexes.
The tetrahedral splitting energy is also called \( \mathrm{\Delta E} \), but it is smaller than in octahedral complexes because there are fewer ligands and less repulsion.
Example
State how the five d orbitals split in an octahedral complex.
▶️ Answer / Explanation
In an octahedral complex, the five degenerate d orbitals split into two higher-energy orbitals and three lower-energy orbitals.
Example
Explain why the order of d-orbital energies is reversed in tetrahedral complexes compared with octahedral complexes.
▶️ Answer / Explanation
In tetrahedral complexes, ligands approach between the axes rather than directly along them.
As a result, the d orbitals that lie between the axes experience greater repulsion and become higher in energy.
This reverses the splitting pattern compared with octahedral complexes.
Colour of Transition Element Compounds
Many compounds of transition elements are coloured. This can be explained by the presence of non-degenerate d orbitals and the absorption of visible light.
Non-degenerate d Orbitals in Complexes

- When ligands bond to a transition metal ion, the five degenerate d orbitals split into two sets with different energies.
- This splitting creates an energy gap, \( \mathrm{\Delta E} \), between the lower- and higher-energy d orbitals.
Absorption of Light
When white light passes through a transition metal compound:

- An electron in a lower-energy d orbital can absorb energy
- The electron is promoted to a higher-energy d orbital
- The energy absorbed matches the splitting energy, \( \mathrm{\Delta E} \)
Because \( \mathrm{\Delta E} \) corresponds to energy in the visible region of the electromagnetic spectrum, light of a specific frequency (or wavelength) is absorbed.
Observed Colour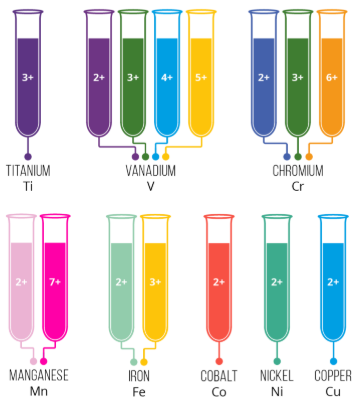
The colour seen is the complementary colour of the light absorbed.
For example, if red light is absorbed, the compound appears green or blue-green.
Why Transition Elements Are Coloured
- They have partially filled d orbitals
- Ligand bonding causes these d orbitals to become non-degenerate
- Electrons can be promoted between these orbitals by absorbing visible light
Elements or ions with full or empty d subshells (such as \( \mathrm{Zn^{2+}} \)) do not usually form coloured compounds.
Example
Explain why many transition metal compounds are coloured.
▶️ Answer / Explanation
Ligand bonding causes the d orbitals of a transition metal ion to split into two non-degenerate energy levels.
Electrons can absorb light of a specific frequency and be promoted from a lower- to a higher-energy d orbital.
This absorption occurs in the visible region, so the compound appears coloured.
Example
Explain why changing the ligand can change the colour of a transition metal complex.
▶️ Answer / Explanation
Different ligands cause different extents of d-orbital splitting, changing the value of \( \mathrm{\Delta E} \).
As a result, light of a different frequency is absorbed when electrons are promoted between non-degenerate d orbitals.
This leads to a different complementary colour being observed.
Effect of Ligands on ΔE and Colour of Transition Metal Complexes
Different ligands cause different extents of d-orbital splitting. This affects the size of the crystal field splitting energy, \( \mathrm{\Delta E} \), the frequency of light absorbed, and therefore the colour observed.
Effect of Ligands on \( \mathrm{\Delta E} \)
Ligands differ in how strongly they interact with the metal ion.
As a result:
- Strong-field ligands cause a larger \( \mathrm{\Delta E} \)
- Weak-field ligands cause a smaller \( \mathrm{\Delta E} \)
Examples (qualitative only):
- \( \mathrm{CN^-} \) and \( \mathrm{NH_3} \) → larger \( \mathrm{\Delta E} \)
- \( \mathrm{H_2O} \) → intermediate \( \mathrm{\Delta E} \)
- \( \mathrm{Cl^-} \) → smaller \( \mathrm{\Delta E} \)
Effect on Frequency of Light Absorbed
The energy absorbed by an electron moving between non-degenerate d orbitals is equal to \( \mathrm{\Delta E} \).

Since energy and frequency are directly proportional:
- Higher \( \mathrm{\Delta E} \) → higher frequency absorbed
- Lower \( \mathrm{\Delta E} \) → lower frequency absorbed
Effect on Observed Colour
The colour observed is the complementary colour of the light absorbed.

Therefore:
- Higher frequency absorption (violet/blue light) → observed colour is yellow/orange
- Lower frequency absorption (red/orange light) → observed colour is blue/green
Overall Qualitative Summary
- Different ligands cause different \( \mathrm{\Delta E} \) values
- \( \mathrm{\Delta E} \) determines the frequency of light absorbed
- The absorbed light determines the complementary colour observed
Example
Explain why a copper(II) complex containing ammonia is a different colour from one containing water.
▶️ Answer / Explanation
Ammonia is a stronger ligand than water.
It causes a larger \( \mathrm{\Delta E} \), so higher-frequency light is absorbed.
As a result, a different complementary colour is observed.
Example
Predict how replacing water ligands with chloride ligands would affect the colour of an octahedral transition metal complex.
▶️ Answer / Explanation
Chloride is a weaker ligand than water.
This decreases \( \mathrm{\Delta E} \), so lower-frequency light is absorbed.
The observed colour therefore shifts towards the blue/green region.
Ligand Exchange and Colour Changes in Transition Metal Complexes
Ligand exchange reactions in transition metal complexes often lead to distinct colour changes. This is because different ligands cause different values of crystal field splitting energy, \( \mathrm{\Delta E} \), so different frequencies of light are absorbed.

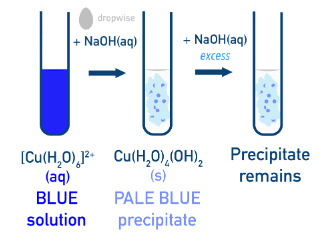
Copper(II) Ion Complexes Copper(II) with Water In aqueous solution, copper(II) ions form the pale blue octahedral complex:
Water is a moderate-field ligand, so the value of \( \mathrm{\Delta E} \) is moderate and light in the visible region is absorbed, giving a pale blue colour. Copper(II) with Hydroxide Ions Addition of hydroxide ions results in formation of a pale blue precipitate:
This occurs because hydroxide ions deprotonate coordinated water molecules. The colour change is associated with loss of the original aqua complex. Copper(II) with Ammonia With excess ammonia, ligand exchange occurs and a deep blue solution forms:
Ammonia is a stronger ligand than water, so it causes a larger \( \mathrm{\Delta E} \). Higher-frequency light is absorbed, and the complementary deep blue colour is observed. | Cobalt(II) Ion Complexes Cobalt(II) with Water In aqueous solution, cobalt(II) ions form a pink octahedral complex:
\( \mathrm{[Co(H_2O)_6]^{2+}} \) Water ligands give a particular value of \( \mathrm{\Delta E} \), leading to absorption of green light and a pink appearance. Cobalt(II) with Chloride Ions In the presence of a high concentration of chloride ions, ligand exchange occurs:
\( \mathrm{[Co(H_2O)_6]^{2+} + 4Cl^- \rightleftharpoons [CoCl_4]^{2-} + 6H_2O} \) The colour changes from pink to blue. Chloride ions are weaker ligands than water, so \( \mathrm{\Delta E} \) decreases. Lower-frequency (red/orange) light is absorbed, producing the blue colour observed. Cobalt(II) with Ammonia Ammonia initially causes precipitation of cobalt(II) hydroxide. In excess ammonia and air, ligand exchange and oxidation lead to ammine complexes with different colours.
|
Explanation Linking Ligand Exchange and Colour
- Ligand exchange replaces one ligand with another of different field strength
- This changes the value of \( \mathrm{\Delta E} \)
- Different frequencies of visible light are absorbed
- The complementary colour observed therefore changes
Example
Explain why adding excess ammonia to a copper(II) aqueous solution changes its colour.
▶️ Answer / Explanation
Ammonia replaces water ligands in a ligand exchange reaction.
Ammonia is a stronger ligand, increasing \( \mathrm{\Delta E} \).
Higher-frequency light is absorbed, so the solution appears deep blue.
Example
Describe and explain the colour change when concentrated hydrochloric acid is added to aqueous cobalt(II) chloride.
▶️ Answer / Explanation
The pink \( \mathrm{[Co(H_2O)_6]^{2+}} \) complex undergoes ligand exchange to form the blue \( \mathrm{[CoCl_4]^{2-}} \) complex.
Chloride ions are weaker ligands than water, so \( \mathrm{\Delta E} \) decreases.
Lower-frequency light is absorbed, resulting in a blue colour.