CIE AS/A Level Chemistry 28.4 Stereoisomerism in transition element complexes Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 28.4 Stereoisomerism in transition element complexes Study Notes – New Syllabus
CIE AS/A Level Chemistry 28.4 Stereoisomerism in transition element complexes Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
associated with bidentate
ligands:
(a) geometrical (cis/trans) isomerism, e.g. square planar such as [Pt(NH₃)₂Cl₂] and octahedral such as
[Co(NH₃)₄(H₂O)₂]²⁺ and [Ni(H₂NCH₂CH₂NH₂)₂(H₂O)₂]²⁺
(b) optical isomerism, e.g. [Ni(H₂NCH₂CH₂NH₂)₃]²⁺ and
[Ni(H₂NCH₂CH₂NH₂)₂(H₂O)₂]²⁺deduce the overall polarity of complexes such as those described in 28.4.1(a) and 28.4.1(b)
Stereoisomerism in Transition Metal Complexes
Stereoisomerism occurs when compounds have the same structural formula but differ in the three-dimensional arrangement of atoms in space. Transition metal complexes commonly show stereoisomerism due to their fixed geometries and the presence of different ligands.
Types of Stereoisomerism in Complexes
- Geometrical (cis/trans) isomerism
- Optical isomerism
(a) Geometrical (cis/trans) Isomerism
Geometrical isomerism occurs when ligands occupy different positions relative to each other around the central metal ion.
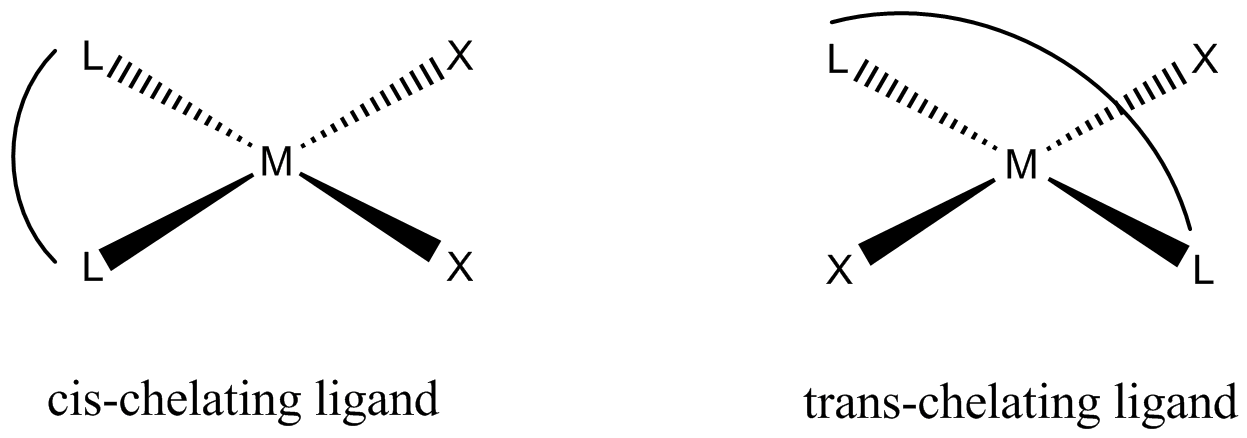
- cis → similar ligands adjacent (90° apart)
- trans → similar ligands opposite (180° apart)
Square Planar Complexes
Square planar complexes with the formula \( \mathrm{MA_2B_2} \) show cis/trans isomerism.
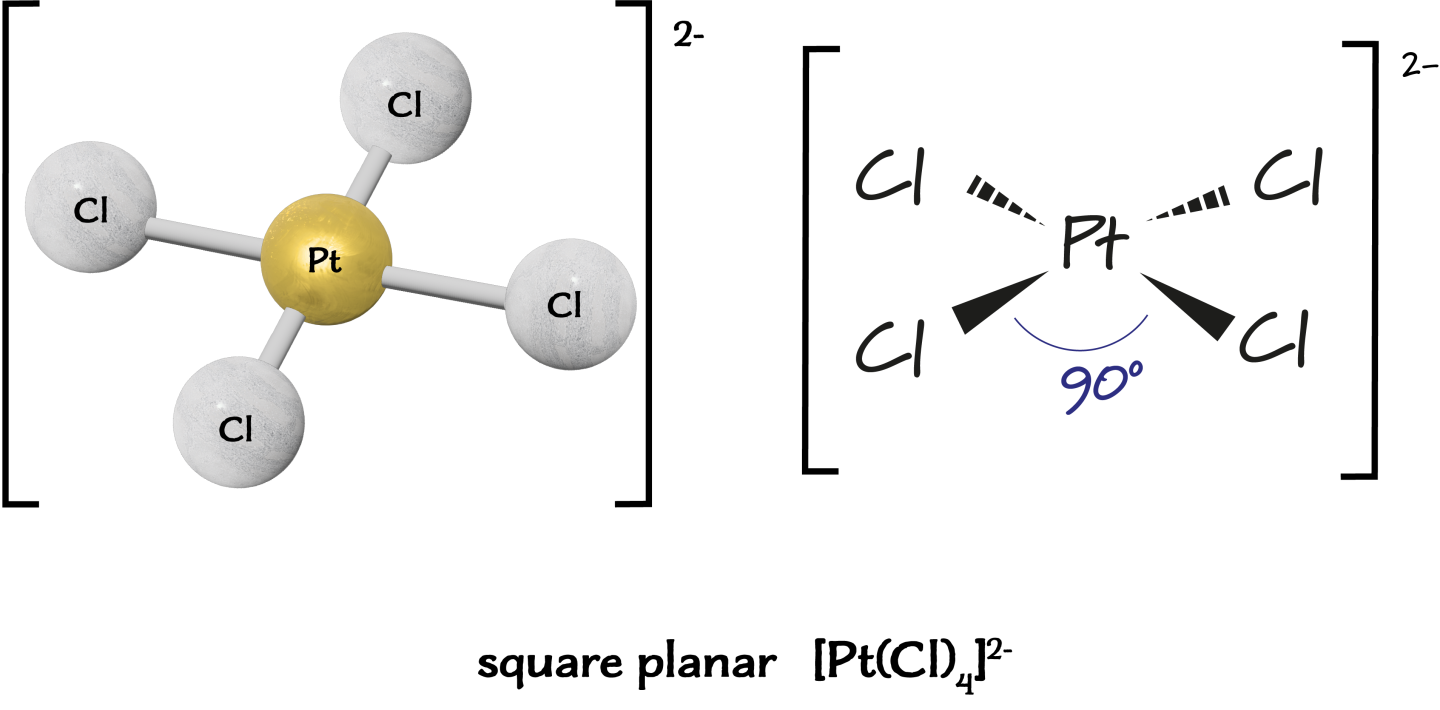
Example:
\( \mathrm{[Pt(NH_3)_2Cl_2]} \)
- cis-[Pt(NH₃)₂Cl₂] → two \( \mathrm{Cl^-} \) ligands adjacent
- trans-[Pt(NH₃)₂Cl₂] → two \( \mathrm{Cl^-} \) ligands opposite
Octahedral Complexes
Octahedral complexes can also show geometrical isomerism.

Example:
\( \mathrm{[Co(NH_3)_4(H_2O)_2]^{2+}} \)
- cis → two \( \mathrm{H_2O} \) ligands adjacent
- trans → two \( \mathrm{H_2O} \) ligands opposite
Geometrical Isomerism with Bidentate Ligands
Bidentate ligands occupy two coordination sites and restrict ligand positions.
Example:
\( \mathrm{[Ni(en)_2(H_2O)_2]^{2+}} \)
- cis form exists
- trans form exists
Here, the two water ligands can be adjacent or opposite.
(b) Optical Isomerism
Optical isomerism occurs when a complex:
- Has no plane of symmetry
- Exists as non-superimposable mirror images (enantiomers)
Each optical isomer rotates plane-polarised light in opposite directions.
Optical Isomerism with Bidentate Ligands
Octahedral complexes containing only bidentate ligands often show optical isomerism.
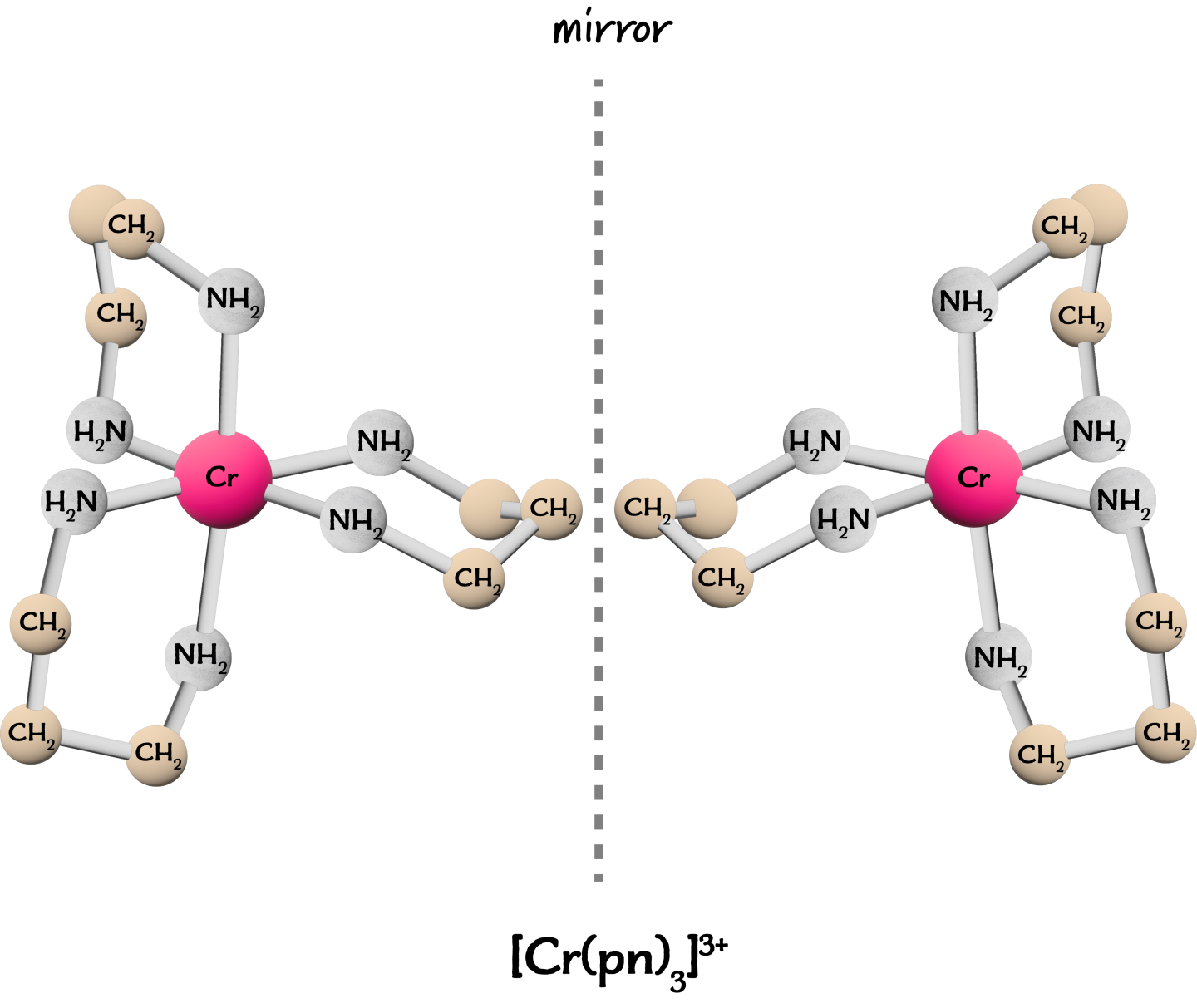
Example:
\( \mathrm{[Ni(en)_3]^{2+}} \)
This complex exists as two optical isomers that are mirror images and cannot be superimposed.
Optical Isomerism with Mixed Ligands
Example:
\( \mathrm{[Ni(en)_2(H_2O)_2]^{2+}} \)
The cis form is optically active because it lacks a plane of symmetry. The trans form is not optically active.
Example
State the type of stereoisomerism shown by \( \mathrm{[Pt(NH_3)_2Cl_2]} \).
▶️ Answer / Explanation
It shows geometrical (cis/trans) isomerism.
Example
Explain why \( \mathrm{[Ni(en)_3]^{2+}} \) shows optical isomerism.
▶️ Answer / Explanation
The complex contains three bidentate ligands arranged octahedrally.
This arrangement has no plane of symmetry and exists as two non-superimposable mirror images.
Therefore, it shows optical isomerism.
Overall Polarity of Transition Metal Complexes
- The overall polarity of a transition metal complex depends on its three-dimensional shape and the arrangement of ligands around the central metal ion.
- Polarity is determined by whether the individual bond dipoles cancel out or reinforce each other.
Key Principle
- A complex is non-polar if it has a symmetrical arrangement of ligands so that bond dipoles cancel.
- A complex is polar if it has an unsymmetrical arrangement of ligands so that bond dipoles do not cancel.
(a) Complexes Showing Geometrical (cis/trans) Isomerism
Square Planar: \( \mathrm{[Pt(NH_3)_2Cl_2]} \)
- trans-[Pt(NH₃)₂Cl₂] is non-polar
- The two \( \mathrm{Cl^-} \) ligands are opposite each other
- Bond dipoles cancel due to symmetry
- cis-[Pt(NH₃)₂Cl₂] is polar
- The \( \mathrm{Cl^-} \) ligands are adjacent
- Dipoles do not cancel
Octahedral: \( \mathrm{[Co(NH_3)_4(H_2O)_2]^{2+}} \)
- trans isomer is non-polar
- Identical ligands are opposite each other, giving symmetry
- cis isomer is polar
- Unequal ligand arrangement leads to incomplete dipole cancellation
Octahedral with Bidentate Ligands: \( \mathrm{[Ni(en)_2(H_2O)_2]^{2+}} \)
- trans isomer is generally non-polar due to symmetry
- cis isomer is polar because ligand positions are unsymmetrical
(b) Complexes Showing Optical Isomerism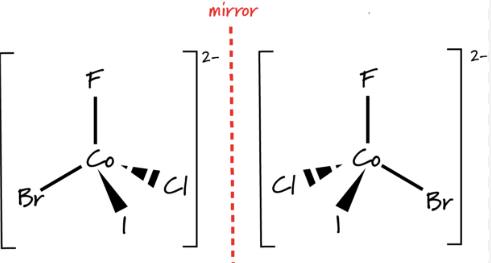
\( \mathrm{[Ni(en)_3]^{2+}} \)
- Contains three bidentate ligands arranged octahedrally
- Has no plane of symmetry
- Forms two optical isomers (enantiomers)
- Each optical isomer is polar
\( \mathrm{[Ni(en)_2(H_2O)_2]^{2+}} \)
- cis form is polar and optically active
- trans form is non-polar and optically inactive
Example
Deduce whether cis- or trans-\( \mathrm{[Pt(NH_3)_2Cl_2]} \) is polar.
▶️ Answer / Explanation
The cis isomer is polar because the ligand arrangement is unsymmetrical.
The trans isomer is non-polar because bond dipoles cancel.
Example
Explain why \( \mathrm{[Ni(en)_3]^{2+}} \) is polar.
▶️ Answer / Explanation
The complex has no plane of symmetry and exists as non-superimposable mirror images.
As a result, bond dipoles do not cancel and each optical isomer is polar.
