CIE AS/A Level Chemistry 29.1 Formulae, functional groups and the naming of organic compounds Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 29.1 Formulae, functional groups and the naming of organic compounds Study Notes – New Syllabus
CIE AS/A Level Chemistry 29.1 Formulae, functional groups and the naming of organic compounds Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
understand that the compounds in the table on page 47 contain a functional group which dictates their
physical and chemical propertiesinterpret and use the general, structural, displayed and skeletal formulas of the classes of compound stated in the table on page 47
understand and use systematic nomenclature of simple aliphatic organic molecules (including cyclic compounds containing a single ring of up to six carbon atoms) with functional groups detailed in the table on page 47 (up to six carbon atoms (six plus six for esters and amides, straight chains only for esters and nitriles))
understand and use systematic nomenclature of simple aromatic molecules with one benzene ring and one or more simple substituents, for example 3-nitrobenzoic acid or 2,4,6-tribromophenol
Organic Compounds and Functional Groups
Organic compounds can be classified into groups based on the functional group they contain. The compounds shown in the table each contain a specific functional group, which dictates their physical and chemical properties.
Functional Groups
A functional group is an atom or group of atoms within a molecule that determines the characteristic reactions of that molecule.
Although the rest of the molecule may vary, compounds containing the same functional group show similar behaviour.
Functional Groups in A Level Organic Chemistry
| Homologous series | Name of functional group | Structural formula of functional group | Displayed formula* | Skeletal formula | Name |
|---|---|---|---|---|---|
| arene | arene | 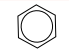 | n/a |  | benzene |
| halogenoarene | halogen |  | n/a |  | chlorobenzene (when X = Cl) |
| phenol | phenol |  | n/a |  | phenol |
| acyl chloride | acyl chloride |  | 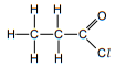 |  | propanoyl chloride |
| amine (secondary and tertiary) | amine |  | 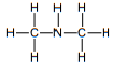 | naming not required | |
| amide (primary, secondary and tertiary) | amide |  | 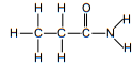 |  | propanamide |
| amino acid | amine and carboxyl |  |  | 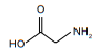 | 2-aminoethanoic acid |
Chemical Properties
The chemical properties of an organic compound depend on the functional group present, as this is the part of the molecule involved in reactions.
- acyl chlorides are highly reactive towards nucleophiles
- amines behave as bases
- phenols show weak acidic behaviour
Physical Properties
The physical properties of compounds are influenced by the functional group through polarity and intermolecular forces.
- functional groups such as –OH and –NH₂ allow hydrogen bonding
- hydrogen bonding increases boiling point and water solubility
- non-polar groups, such as in arenes, lead to low solubility in water
For example, compounds containing an –OH group can form hydrogen bonds, while compounds containing an acyl chloride group are highly reactive.
Example
Phenol and benzene both contain a benzene ring. Phenol has a higher boiling point than benzene. Explain this difference in terms of functional groups.
▶️ Answer / Explanation
Phenol contains an –OH functional group, whereas benzene does not contain a functional group.
The –OH group allows phenol molecules to form hydrogen bonds, resulting in stronger intermolecular forces.
Benzene is non-polar and only experiences London forces, so phenol has a higher boiling point.
Example
Ethylamine and ethanamide have similar molecular masses. However, ethanamide has a much higher melting point. Explain why.
▶️ Answer / Explanation
Ethylamine contains an amine functional group, while ethanamide contains an amide functional group.
Amide groups are more polar and can form multiple hydrogen bonds between molecules.
This results in stronger intermolecular forces in ethanamide, giving it a higher melting point despite a similar molecular mass.
Representing Organic Compounds Using Different Formulae
Organic compounds can be represented in several different ways. You must be able to interpret and use the general, structural, displayed and skeletal formulae for the classes of compound listed in the table.
General Formula
A general formula shows the ratio of atoms in a homologous series and allows the formula of any member to be predicted.![]()
It does not show how atoms are arranged or bonded.
- Alkanes: \( \mathrm{C_{n}H_{2n+2}} \)
- Alkenes: \( \mathrm{C_{n}H_{2n}} \)
- Arenes: \( \mathrm{C_{n}H_{2n-6}} \)
Structural Formula
A structural formula shows how atoms are connected in a molecule but does not show every bond.
It is particularly useful for identifying functional groups, such as \( \mathrm{–COCl} \) in acyl chlorides or \( \mathrm{–CONH_2} \) in amides.
Displayed Formula
A displayed formula shows all atoms and all bonds in the molecule.
Displayed formulae are not usually required when a benzene ring is present, but they may be used for non-aromatic compounds such as amides and amino acids.
Skeletal Formula
A skeletal formula shows the carbon framework of a molecule using lines.
- each line end or corner represents a carbon atom
- hydrogen atoms bonded to carbon are omitted
- heteroatoms such as O, N and Cl are always shown
Skeletal formulae are especially common for benzene-containing compounds such as phenol and halogenoarenes.
Example
The general formula of an arene is \( \mathrm{C_{n}H_{2n-6}} \). Deduce the molecular formula of the arene with eight carbon atoms and write its structural formula.
▶️ Answer / Explanation
Substitute \( \mathrm{n = 8} \) into the general formula:
\( \mathrm{C_8H_{2(8)-6} = C_8H_{10}} \)
A suitable structural formula is:
\( \mathrm{C_6H_5CH_2CH_3} \) (ethylbenzene).
Example
The skeletal formula of a compound shows a benzene ring with a –COCl group attached.
(a) Identify the class of compound.
(b) Write the structural formula and name the compound.
▶️ Answer / Explanation
(a) The –COCl group identifies the compound as an acyl chloride.
(b) The structural formula is:
\( \mathrm{C_6H_5COCl} \)
The compound is named benzoyl chloride.
Example
The skeletal formula of a compound shows a benzene ring with an –OH group attached.
(a) Identify the homologous series.
(b) Write the structural formula and name the compound.
▶️ Answer / Explanation
(a) The –OH group attached to a benzene ring identifies the compound as a phenol.
(b) The structural formula is a benzene ring with an –OH group attached.
The compound is named phenol.
Example
A compound has the molecular formula \( \mathrm{C_2H_5NO_2} \) and its skeletal formula shows both an –NH₂ group and a –COOH group.
(a) Identify the class of compound.
(b) Write a suitable structural formula and name the compound.
▶️ Answer / Explanation
(a) The presence of both an amine group and a carboxylic acid group identifies the compound as an amino acid.
(b) A suitable structural formula is:
\( \mathrm{NH_2CH_2COOH} \)
The compound is named 2-aminoethanoic acid.
Systematic Nomenclature of Simple Aliphatic Organic Molecules
You must be able to understand and use systematic nomenclature to name and interpret simple aliphatic organic molecules, including:
- straight-chain and branched aliphatic compounds
- cyclic compounds containing a single ring of up to six carbon atoms
- molecules containing the functional groups listed in the table
The total number of carbon atoms is limited to six, except for:
- esters and amides: up to six carbons on each side
- esters and nitriles: straight chains only
Principles of Systematic Naming
Systematic nomenclature assigns each molecule a unique name based on its structure, ensuring that the name clearly indicates:

- the longest carbon chain
- the functional group present
- the position of the functional group
- any substituents and their positions
Aliphatic and Cyclic Compounds
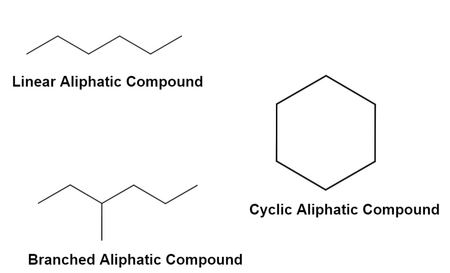
Aliphatic compounds contain carbon atoms arranged in straight or branched chains.
Cyclic compounds are named using the prefix cyclo–, followed by the parent alkane name.
Example: a six-carbon ring containing only single bonds is named cyclohexane.
Functional Group Suffixes
- alkanes: –ane
- alkenes: –ene
- alcohols: –ol
- aldehydes: –al
- ketones: –one
- carboxylic acids: –oic acid
- esters: –oate
- amines: –amine
- amides: –amide
- nitriles: –nitrile
Example
Name the following compound: a straight-chain molecule with four carbon atoms and a carboxylic acid group.
▶️ Answer / Explanation
The parent chain contains four carbon atoms, so the root name is butan–.
The functional group is a carboxylic acid, which uses the suffix –oic acid.
The compound is named butanoic acid.
Example
A molecule has the structural formula \( \mathrm{CH_3CH_2COOCH_2CH_3} \).
Name the compound and explain how the name is constructed.
▶️ Answer / Explanation
The molecule is an ester.
The alkyl group attached to the oxygen is ethyl.
The acid part contains three carbon atoms, giving propanoate.
The compound is named ethyl propanoate.
Each side of the ester contains fewer than six carbon atoms and both chains are straight, satisfying the specification.
Systematic Nomenclature of Simple Aromatic Molecules
You must be able to understand and use systematic nomenclature to name simple aromatic molecules containing:
- one benzene ring
- one or more simple substituents
- functional groups listed in the specification

Examples include compounds such as 3-nitrobenzoic acid and 2,4,6-tribromophenol.
The Benzene Ring as the Parent Structure
In aromatic compounds, the benzene ring forms the parent structure.
The parent name depends on the highest-priority functional group attached to the ring.
- –COOH → benzoic acid
- –OH → phenol
- –NH₂ → phenylamine
- –NO₂, –Br, –Cl, –CH₃ → named as substituents on benzene
Numbering the Benzene Ring
The carbon atom bonded to the principal functional group is assigned position 1.
The ring is numbered to give substituents the lowest possible set of locants.
Numbers are used instead of ortho-, meta-, and para- in systematic nomenclature.
Naming Multiple Substituents
- Use prefixes such as di–, tri–, tetra– when needed
- Substituents are listed in alphabetical order (ignoring prefixes)
- Each substituent position must be clearly indicated with a number
Example
A benzene ring contains a carboxylic acid group and a nitro group in the 3-position. Name the compound.
▶️ Answer / Explanation
The highest-priority functional group is the carboxylic acid, so the parent name is benzoic acid.
The nitro group is a substituent at position 3.
The compound is named 3-nitrobenzoic acid.
Example
A phenol molecule has bromine atoms attached at the 2-, 4- and 6-positions on the benzene ring. Name the compound.
▶️ Answer / Explanation
The principal functional group is –OH, so the parent compound is phenol.
There are three bromine substituents at positions 2, 4 and 6.
The prefix tri– is used for three identical substituents.
The compound is named 2,4,6-tribromophenol.
