CIE AS/A Level Chemistry 29.2 Characteristic organic reactions Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 29.2 Characteristic organic reactions Study Notes – New Syllabus
CIE AS/A Level Chemistry 29.2 Characteristic organic reactions Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
- understand and use the following terminology associated with types of organic mechanisms:
(a) electrophilic substitution
(b) addition–elimination
Terminology Used in Organic Reaction Mechanisms
Organic reactions can be grouped into different mechanism types. You must be able to understand and use the correct terminology associated with the following mechanisms:
- electrophilic substitution
- addition–elimination
Electrophilic Substitution
Electrophilic substitution is a reaction in which an electrophile replaces a hydrogen atom in an organic molecule.
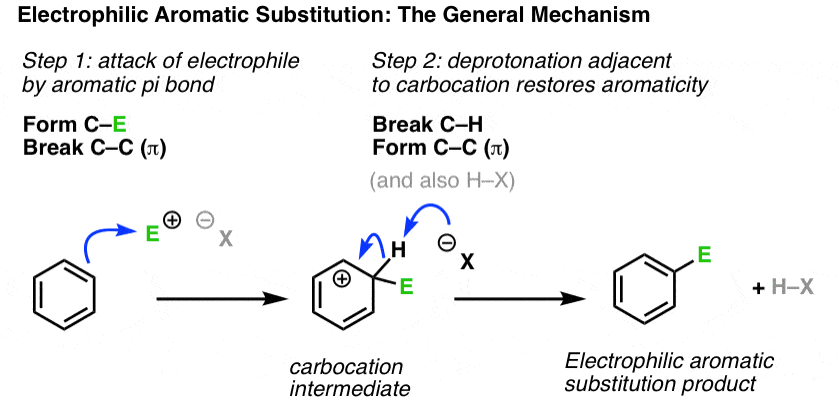
This type of mechanism is characteristic of compounds containing a benzene ring.
The benzene ring has a delocalised π electron system, making it attractive to electrophiles. Substitution occurs rather than addition because addition would destroy the aromatic system.
Key Terminology
- electrophile: an electron-pair acceptor
- substitution: one atom or group is replaced by another
Addition–Elimination
Addition–elimination is a two-step mechanism in which:
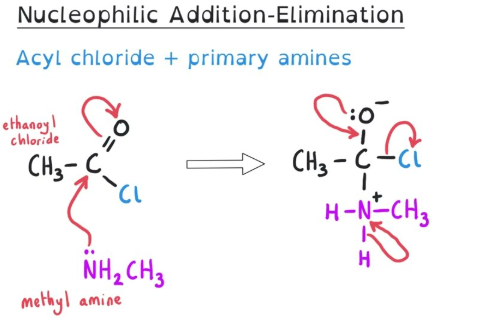
- a nucleophile is first added to a molecule
- a leaving group is then eliminated
This mechanism occurs in compounds containing a carbonyl group bonded to a strongly electronegative atom.
Key Terminology
- nucleophile: an electron-pair donor
- addition: atoms or groups join a molecule
- elimination: removal of a small molecule or ion
Example
Chlorination of benzene occurs via electrophilic substitution rather than addition. Explain why.
▶️ Answer / Explanation
Benzene contains a delocalised π electron system.
Addition reactions would break this system and make the molecule less stable.
In electrophilic substitution, a hydrogen atom is replaced by an electrophile, so the aromatic structure is preserved.
Example
An acyl chloride reacts with ammonia to form an amide. Identify the type of mechanism and justify your answer.
▶️ Answer / Explanation
The reaction proceeds via an addition–elimination mechanism.
Ammonia acts as a nucleophile and adds to the electron-deficient carbonyl carbon.
This is followed by elimination of the chloride ion as a leaving group.
The overall reaction involves addition followed by elimination.
