CIE AS/A Level Chemistry 29.3 Shapes of aromatic organic molecules Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 29.3 Shapes of aromatic organic molecules Study Notes – New Syllabus
CIE AS/A Level Chemistry 29.3 Shapes of aromatic organic molecules Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
- describe and explain the shape of benzene and other aromatic molecules, including sp² hybridisation, in terms of σ bonds and a delocalised π system
Shape and Bonding in Benzene and Aromatic Molecules
Aromatic molecules such as benzene have a distinctive planar shape and unusual bonding. You must be able to describe and explain this structure in terms of sp² hybridisation, σ bonds and a delocalised π system.
Structure and Shape of Benzene
Benzene is a planar hexagonal molecule in which all six carbon atoms lie in the same plane. Each bond angle is 120°, giving a regular hexagonal shape.
All carbon–carbon bond lengths in benzene are equal, with values intermediate between a single and double bond.
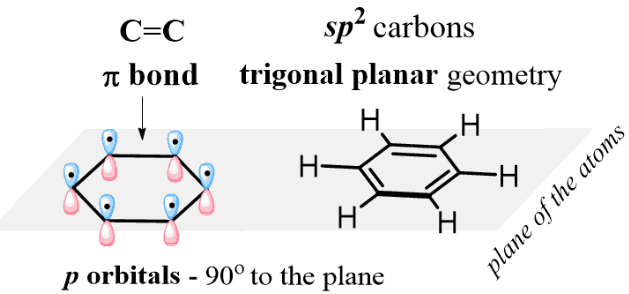
sp² Hybridisation
Each carbon atom in benzene is sp² hybridised.
This means that one s orbital and two p orbitals combine to form three equivalent sp² hybrid orbitals lying in a plane.

- two sp² orbitals form σ bonds with neighbouring carbon atoms
- one sp² orbital forms a σ bond with a hydrogen atom
This arrangement explains the trigonal planar geometry and 120° bond angles.
σ Bonds in Benzene
A σ bond is formed by end-on overlap of orbitals.
In benzene:
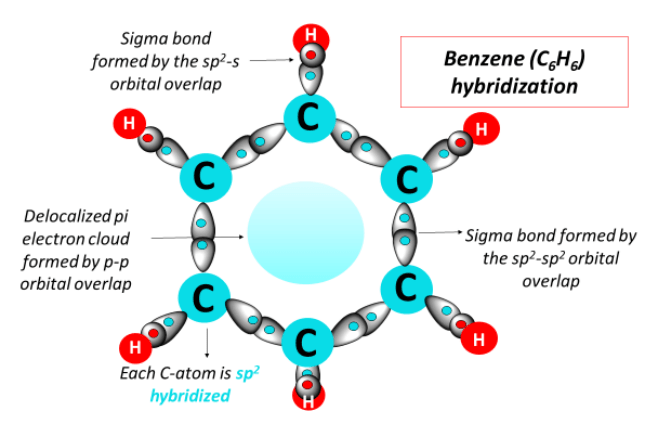
- each C–C bond is a σ bond formed by overlap of sp² orbitals
- each C–H bond is a σ bond formed by overlap of a carbon sp² orbital and a hydrogen 1s orbital
Delocalised π System
Each carbon atom has one unhybridised p orbital perpendicular to the plane of the ring.
These p orbitals overlap sideways around the ring, forming a delocalised π system above and below the plane of the molecule.
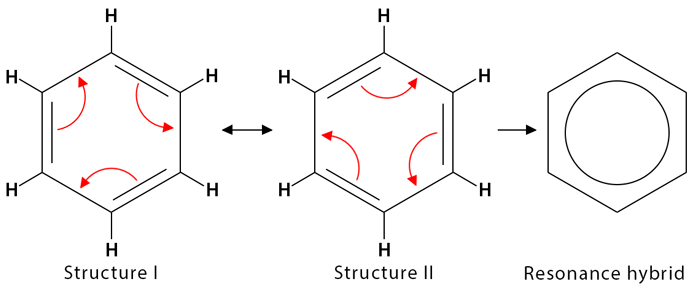
The π electrons are not confined between individual pairs of carbon atoms and are shared across the entire ring.
Consequences of Delocalisation
- all C–C bonds have the same length and strength
- benzene is unusually stable compared to alkenes
- benzene undergoes electrophilic substitution rather than addition reactions
Addition reactions would disrupt the delocalised π system and reduce stability.
Example
Explain why all carbon–carbon bond lengths in benzene are equal.
▶️ Answer / Explanation
Each carbon atom in benzene is sp² hybridised and has an unhybridised p orbital.
The p orbitals overlap to form a delocalised π system around the ring.
As the π electrons are shared across all six carbon atoms, all C–C bonds have equal length, intermediate between single and double bonds.
Example
Describe the bonding in benzene, including reference to sp² hybridisation, σ bonds and π bonding.
▶️ Answer / Explanation
Each carbon atom in benzene is sp² hybridised, forming three sp² orbitals arranged at 120° in a plane.
Two sp² orbitals overlap with neighbouring carbon atoms to form C–C σ bonds, and the third overlaps with a hydrogen atom to form a C–H σ bond.
The remaining unhybridised p orbitals on each carbon overlap sideways to form a delocalised π system above and below the plane of the ring.
This delocalisation explains the equal bond lengths and high stability of benzene.
