CIE AS/A Level Chemistry 29.4 Isomerism: optical Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 29.4 Isomerism: optical Study Notes – New Syllabus
CIE AS/A Level Chemistry 29.4 Isomerism: optical Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
understand that enantiomers have identical physical and chemical properties apart from their ability to rotate plane polarised light and their potential biological activity
understand and use the terms optical active and racemic mixture
describe the effect on plane polarised light of the two optical isomers of a single substance
explain the relevance of chirality to the synthetic preparation of drug molecules including:
(a) the potential different biological activity of the two enantiomers
(b) the need to separate a racemic mixture into two pure enantiomers
(c) the use of chiral catalysts to produce a single pure optical isomer(Candidates should appreciate that compounds can contain more than one chiral centre, but knowledge
of meso compounds and nomenclature such as diastereoisomers is not required.)
Properties of Enantiomers
Enantiomers are a type of stereoisomer that exist as non-superimposable mirror images. You must understand that enantiomers have identical physical and chemical properties, except for their effect on plane polarised light and their biological activity.
![]()
Identical Properties
Enantiomers have identical physical properties such as:
- melting point
- boiling point
- density
- solubility in a given solvent
They also have identical chemical properties and react at the same rate with achiral reagents.
Optical Activity
The key physical difference between enantiomers is their effect on plane polarised light.
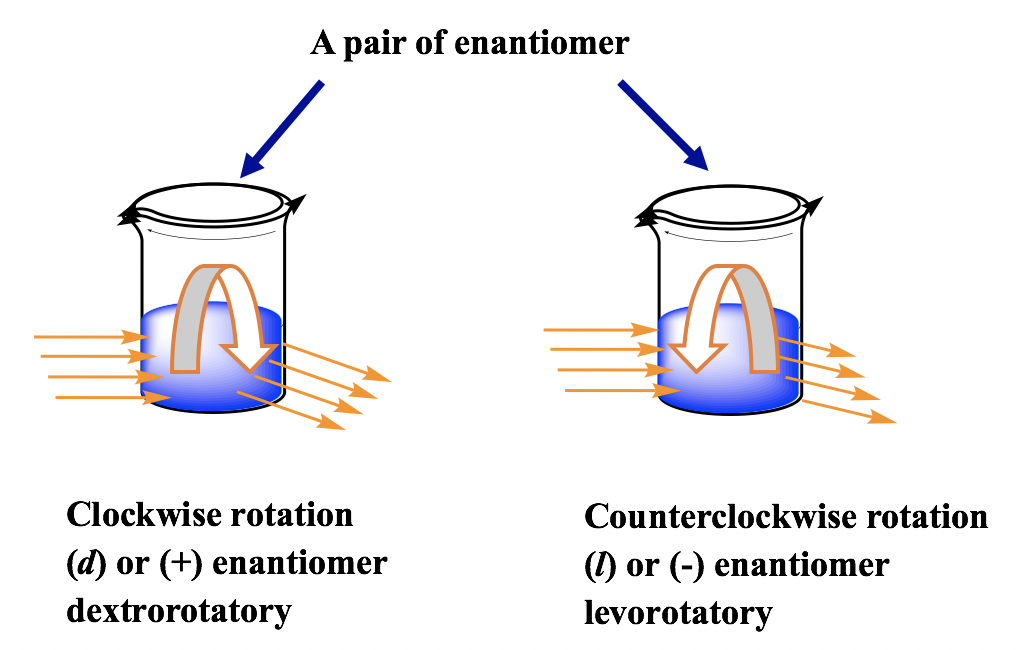
Each enantiomer rotates plane polarised light by the same amount but in opposite directions.
- + (or d) enantiomer: rotates light clockwise
- − (or l) enantiomer: rotates light anticlockwise
A racemic mixture contains equal amounts of both enantiomers and does not rotate plane polarised light overall.
Biological Activity
Although enantiomers have identical chemistry, they may behave differently in biological systems.
This is because enzymes and receptors are chiral and may only interact effectively with one enantiomer.
As a result, one enantiomer may be beneficial, inactive, or harmful, while the other is not.
Example
Two enantiomers have identical melting points and react at the same rate with sodium hydroxide. State one physical property by which they can be distinguished.
▶️ Answer / Explanation
They can be distinguished by their effect on plane polarised light.
One enantiomer rotates plane polarised light clockwise, while the other rotates it anticlockwise by the same amount.
Example
Explain why one enantiomer of a drug may be biologically active while the other is inactive, even though they have identical physical and chemical properties.
▶️ Answer / Explanation
Enzymes and receptors in biological systems are chiral.
Only one enantiomer may fit correctly into the active site of a receptor or enzyme.
The other enantiomer does not interact in the same way and may therefore be inactive or have a different biological effect.
Optically Active Compounds and Racemic Mixtures
Some organic compounds affect plane polarised light. You must be able to understand and use correctly the terms optically active and racemic mixture.
Optically Active
An optically active substance is one that rotates plane polarised light.

A compound is optically active if it contains a single enantiomer or an unequal mixture of enantiomers.
- rotation may be clockwise (d or +)
- rotation may be anticlockwise (l or −)
The direction of rotation depends on the enantiomer present, not on the R/S configuration.
Racemic Mixture
A racemic mixture contains equal amounts of the two enantiomers of a chiral compound.

Although each enantiomer is optically active, a racemic mixture is optically inactive.
This is because the rotations of plane polarised light by each enantiomer are equal in magnitude but opposite in direction, so they cancel out.
Key Comparison
- optically active substance: rotates plane polarised light
- racemic mixture: does not rotate plane polarised light overall
Example
State whether each of the following samples is optically active or optically inactive:
(a) a pure enantiomer
(b) a racemic mixture
▶️ Answer / Explanation
(a) A pure enantiomer is optically active because it rotates plane polarised light.
(b) A racemic mixture is optically inactive because the rotations caused by each enantiomer cancel out.
Example
A reaction produces a mixture containing equal amounts of two enantiomers.
(a) Name this type of mixture.
(b) State whether it is optically active and explain why.
▶️ Answer / Explanation
(a) The mixture is a racemic mixture.
(b) It is optically inactive.
Each enantiomer rotates plane polarised light by the same amount in opposite directions, so there is no overall rotation.
Effect of Optical Isomers on Plane Polarised Light
Optical isomers (enantiomers) affect plane polarised light in a characteristic way. You must be able to describe the effect of the two optical isomers of a single substance on plane polarised light.
Plane Polarised Light
Plane polarised light vibrates in one plane only.

When plane polarised light passes through a solution containing a chiral compound, the plane of polarisation may be rotated.
Effect of the Two Optical Isomers
The two optical isomers of the same substance rotate plane polarised light by the same amount but in opposite directions.
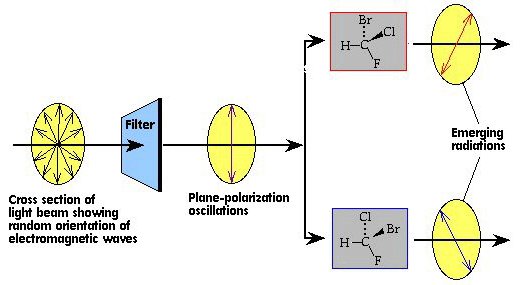
- one enantiomer rotates light clockwise (d or +)
- the other rotates light anticlockwise (l or −)
Apart from this difference, the two optical isomers have identical physical and chemical properties.
Key Exam Statement
For a given substance, the two optical isomers rotate plane polarised light by equal magnitudes in opposite directions.
Example
Describe the effect on plane polarised light of the two enantiomers of the same chiral compound.
▶️ Answer / Explanation
One enantiomer rotates plane polarised light clockwise.
The other enantiomer rotates plane polarised light anticlockwise by the same amount.
Example
A solution of one optical isomer rotates plane polarised light by 12° clockwise. Describe the effect of the other optical isomer under the same conditions.
▶️ Answer / Explanation
The other optical isomer will rotate plane polarised light by 12° anticlockwise.
This is because the two enantiomers rotate plane polarised light by equal amounts in opposite directions.
Relevance of Chirality in the Synthesis of Drug Molecules
Many drug molecules are chiral. Chirality is highly relevant in drug synthesis because different enantiomers of the same compound can behave very differently in the body. You must be able to explain the importance of chirality in terms of:

- different biological activity of enantiomers
- the need to separate racemic mixtures
- the use of chiral catalysts to produce a single optical isomer
(a) Different Biological Activity of Enantiomers
Although enantiomers have identical physical and chemical properties, they can have different biological effects.

This is because biological systems (such as enzymes and receptors) are chiral. As a result, one enantiomer may:
- fit a receptor and be biologically active
- be less active or inactive
- cause unwanted side effects
The other enantiomer may interact differently or not at all with the same receptor.
(b) Separation of Racemic Mixtures
Many synthetic reactions produce a racemic mixture, containing equal amounts of both enantiomers.

If only one enantiomer has the desired biological effect, the racemic mixture must be separated into two pure enantiomers.
This ensures that:
- the correct enantiomer is administered
- side effects from the unwanted enantiomer are reduced or avoided
- dosage can be controlled accurately
(c) Use of Chiral Catalysts
A chiral catalyst is a catalyst that causes one enantiomer to be formed in preference to the other.
Using a chiral catalyst allows the reaction to produce a single pure optical isomer rather than a racemic mixture.
This is advantageous because it:
- reduces the need for separation processes
- increases yield of the desired enantiomer
- makes drug manufacture more efficient and cost-effective
Additional Consideration
Drug molecules may contain more than one chiral centre, increasing the number of possible stereoisomers.
However, detailed knowledge of meso compounds or terminology such as diastereoisomers is not required.
Example
Explain why two enantiomers of the same drug can have different biological effects.
▶️ Answer / Explanation
Enzymes and receptors in the body are chiral.
One enantiomer may fit correctly into a receptor and be biologically active.
The other enantiomer may not fit the receptor in the same way and may be inactive or cause side effects.
Example
A synthetic route to a chiral drug produces a racemic mixture.
(a) Explain why this is undesirable.
(b) Explain how the use of a chiral catalyst could overcome this problem.
▶️ Answer / Explanation
(a) A racemic mixture contains equal amounts of two enantiomers.
Only one enantiomer may have the desired biological activity, while the other may be inactive or cause side effects.
(b) A chiral catalyst causes one enantiomer to form in preference to the other.
This produces a single pure optical isomer, removing the need to separate the racemic mixture.
